Intas licenses Helnius’s antibody for marketing in Europe and India
Pharmaceutical Technology
OCTOBER 30, 2023
Helinus will receive €42m upfront and will be in line to receive up to €143m in regulatory and sales-based milestone payments.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Pharmaceutical Technology
JULY 21, 2022
AstraZeneca has signed a deal with the Federal Office of Public Health (FOPH) of Switzerland to deliver over 1,200 doses of antibody therapy, tixagevimab and cilgavimab combination (AZD7442), for Covid-19 prevention and treatment. In June 2020, these antibodies, discovered at Vanderbilt University Medical Center, were licensed to AstraZeneca.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
JULY 4, 2022
Brii Biosciences (Brii Bio) has exercised an option for the acquisition of exclusive development and marketing rights for Vir Biotechnology’s investigational antibody, VIR-3434, for Hepatitis B in Greater China, under a partnership agreement. The mAb is presently in the Phase II development stage.
Pharmaceutical Technology
OCTOBER 19, 2022
Syncromune and Biocytogen Pharmaceuticals’ wholly owned subsidiary Eucure Biopharma have entered an exclusive global licence agreement for OX40 antibody YH002 and two other active ingredients. . Eucure will oversee the production and delivery of the drug, while Syncromune will handle the clinical development and marketing.

Pharmaceutical Technology
NOVEMBER 27, 2022
Biocytogen Pharmaceuticals and ADC Therapeutics have signed an assessment and option agreement for evaluating antibodies against three tumour targets. Under the deal, ADC Therapeutics will receive a licence from Biocytogen to evaluate the latter’s antibodies against the targets.
Pharmaceutical Technology
DECEMBER 26, 2022
LegoChem Biosciences and Amgen have signed a multi-target research collaboration and license agreement to develop antibody-drug conjugates (ADC). Till date, the company has signed a total of 12 ADC licensing deals, worth more than $5bn.
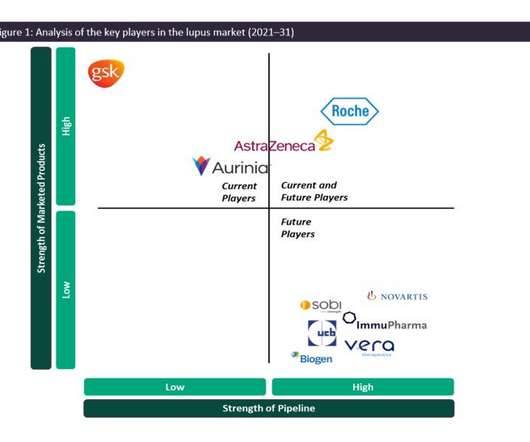
Pharmaceutical Technology
JANUARY 23, 2023
As such, the SLE and LN marketplace is dominated by generics, and GSK’s Benlysta and AstraZeneca’s Saphnelo are the only drugs that have gained marketing approval specifically for SLE in more than 50 years. In previous years, Benlysta has managed to grow the lupus market in terms of value, having generated approximately $492.9m
BioPharma Reporter
APRIL 2, 2024
Ipsen is expanding its oncology pipeline by securing the global licensing rights to an antibody-drug conjugate (ADC) for solid tumors from Sutro Biopharma, its first drug in the category.

BioPharma Reporter
OCTOBER 26, 2023
The biopharmaceutical industry witnessed a 400% growth in total deal value of antibody-drug conjugate (ADC) licensing agreements from 2017-2022 and reached a peak of $16.6 billion in 2022, reveals data and analytics company GlobalData.
Pharmaceutical Technology
MAY 24, 2023
Y-mAbs Therapeutics has received marketing authorisation for Danyelza (naxitamab-gqgk) 40mg/10mL injection from the Brazilian Health Regulatory Agency, Agência Nacional de Vigilância Sanitária, to treat high-risk neuroblastoma. The therapy is given three times a week and repeated every four weeks.
BioPharma Reporter
JUNE 1, 2021
Corbus Pharmaceuticals announced the expansion of its portfolio into immuno-oncology through licensing deals with the University of California San Francisco and Panorama Research Inc for two new monoclonal antibodies (mAbs).
pharmaphorum
DECEMBER 16, 2020
AbbVie is to begin clinical development of an antibody designed to neutralise the SARS-CoV-2 coronavirus after licensing the therapy in from Harbour BioMed and Utrecht University. AbbVie has begun a phase 1 clinical trial of the antibody, with clinical development beginning in the US and expanding into Europe.

BioPharma Reporter
JANUARY 6, 2023
Synaffix, a Netherlands based company providing clinical-stage platform technology for the development of antibody-drug conjugates (ADCs), has signed off on two new licensing deals this week.

Pharmaceutical Technology
MAY 8, 2023
Reddy’s Laboratories have partnered for the development and commercialisation of the anti-PD-1 monoclonal antibody, toripalimab, in 21 countries. The company may also choose to expand the scope to license toripalimab in New Zealand, Australia, and in nine other countries.

pharmaphorum
SEPTEMBER 27, 2022
While a rumoured takeover by Merck & Co has yet to materialise, Seagen is getting on with its own business development, including a just-agreed licensing deal for a cancer immunotherapy developed by Dutch biotech Lava Therapeutics. The post Lava fires up a $700m cancer licensing deal with Seagen appeared first on.

The Pharma Data
JANUARY 11, 2021
a San Diego-based biotechnology company with an array of technology platforms for antibody discovery and optimization, and novel NK and T cell engager generation, today announced licensing of a panel of its anti-SARS-CoV-2 antibody clones to IGM Biosciences for COVID-19 therapy development.

The Pharma Data
JUNE 2, 2022
Boehringer Ingelheim and the Agency for Science, Technology and Research (A*STAR) today announced a global licensing agreement under which Boehringer Ingelheim will obtain exclusive worldwide rights to research, develop and commercialize products based on a panel of innovative, tumor-specific antibodies from A*STAR.
Roots Analysis
JANUARY 21, 2024
With fourteen approved drugs and several drug candidates being evaluated under different stages of development, antibody drug conjugates (ADCs) are now recognized as a potent class of targeted therapeutics. In this context, the role of the linker molecule and the conjugation technology used is pivotal.

Pharmaceutical Technology
JANUARY 19, 2023
Acute myeloid leukemia (AML) is part of a market of blood malignancies that commercial cell therapies have not managed to penetrate yet. However, it is possible to target CD33 in the clinic, as evidenced by the FDA approval of Pfizer’s anti-CD33 antibody-drug conjugate Mylotarg (gemtuzumab ozogamicin) in 2000.

pharmaphorum
APRIL 9, 2021
The FDA says it has uncovered “deficiencies” in the marketing application for Provention Bio’s much-anticipated drug teplizumab for the prevention of type 1 diabetes (T1D) that could delay its review. The post Provention faces delay in FDA review of diabetes prevention antibody appeared first on.

BioPharma Reporter
JANUARY 5, 2023
AstraZeneca and Sanofi said their Biologics License Application (BLA) for nirsevimab has been accepted for review by the US Food and Drug Administration (FDA).

pharmaphorum
JULY 8, 2022
Swedish rare disease specialist Sobi is paying $55 million upfront to license rights to ADC Therapeutics’ lymphoma therapy Zynlonta – approved in the US last year – in Europe and other international markets. The post Sobi bolsters blood division via $435m ADC licensing deal appeared first on.

Pharmaceutical Technology
SEPTEMBER 13, 2022
Gilead’s Trodelvy (sacituzumab govitecan-hziy) is a first-in-class Trop-2 directed antibody-drug conjugate (ADC), with a potent topoisomerase I inhibitor payload. As Trodelvy will have the first-in-class advantage in the HER2-negative setting, dato-DXd would have to demonstrate superior efficacy to dominate this market.

pharmaphorum
JANUARY 4, 2023
The Janssen Pharmaceutical Companies of Johnson & Johnson have submitted a Marketing Authorisation Application (MAA) to the European Medicines Agency (EMA), seeking approval of talquetamab for the treatment of patients with relapsed or refractory multiple myeloma (RRMM). This reduces the timeframe for the MAA to be reviewed.
Pharmaceutical Technology
MAY 12, 2023
On 8 May 2023, China-based Bliss Biopharmaceutical (BlissBio) announced a clinical trial collaboration with Eisai to develop BB-1701, an antibody-drug conjugate (ADC) for multiple cancer types. Seagen has three marketed ADCs as well as two novel ones, one of which, disitamab vedotin, is HER2-directed.

pharmaphorum
APRIL 14, 2022
Epcoritamab is a bispecific antibody which targets CD3 on white blood cells and CD20 on tumour cells, and is designed to encourage an immune response against the cancer. The post AbbVie preps filings for lymphoma bispecific licensed from Genmab appeared first on. billion product at peak. billion product at peak.
pharmaphorum
OCTOBER 18, 2022
Gilead Sciences has made yet another rush into the oncology category, licensing a bispecific antibody from MacroGenics in development as a treatment for CD123-positive blood cancers, including acute myeloid leukaemia (AML) and myelodysplastic syndromes (MDS). Along with the signing fee, there is another $1.7

Pharmaceutical Technology
DECEMBER 19, 2022
With the latest development, Sanofi will licence a NK cell engager programme that acts on B7H3 from the antibody-based NK cell engager therapeutics (ANKET) platform of Innate. On choosing the candidate, the company will oversee the complete development, production and marketing.

Pharmaceutical Technology
APRIL 6, 2023
The designation, under the regulator’s Innovative Licensing and Access Pathway (ILAP), will fast-track a potential route to market for AD04 by providing collaborative opportunities with UK institutes like the National Institute for Health and Care Excellence (NICE).
pharmaphorum
MARCH 29, 2021
The EU looks set to approve emergency use of a third antibody therapy for COVID-19 after its human medicines committee backed use of Celltrion’s regdanvimab at its March meeting. . The EU’s medicines regulator is also reviewing COVID-19 antibodies from AstraZeneca , GlaxoSmithKline/Vir Biotechnology, and Brii Biosciences.

pharmaphorum
FEBRUARY 8, 2022
The FDA has dropped a bomb on Eli Lilly’s marketing application for cancer immunotherapy sintilimab ahead of an advisory committee meeting due to take place on Thursday. Lilly has a long history of developing oncology products, but missed the boat when the market started to shift towards cancer immunotherapies a few years back.

pharmaphorum
JUNE 24, 2021
Armed with a positive mid-stage trial readout in lung cancer, Arcus Biosciences will expand a phase 3 programme for domvanalimab, its anti-TIGIT antibody. Zimberelimab “showed activity similar to that of marketed anti-PD-1 antibodies studied in this setting,” said Arcus’ chief medical officer Bill Grossman.
Pharmaceutical Technology
OCTOBER 7, 2022
A decision on the approval of the Biologics License Application (BLA) is anticipated on 17 November. Additionally, the company granted Sanofi an exclusive right of first negotiation (ROFN) to attain international rights to market teplizumab for T1D indications for a one-time payment of $20m.

pharmaphorum
NOVEMBER 10, 2021
It is competing in the US with rival oral CGRPs, namely AbbVie’s Qulipta (atogepant) which was approved for episodic migraine prevention in September and AbbVie’s Ubrelvy (ubrogepant) in the acute treatment category, as well as several CGRP-targeting antibodies delivered by injection or infusion from Amgen, Eli Lilly, Teva and Lundbeck.
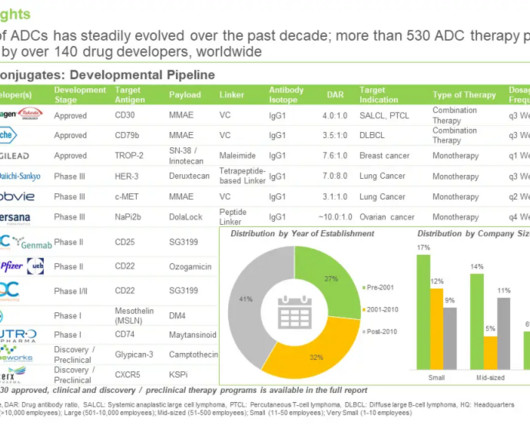
Roots Analysis
FEBRUARY 27, 2024
Currently, product licensing agreements, technology licensing agreements, research and development agreements and clinical trial agreements are the common types of partnerships inked by developers that are currently focused on the development of ADCs pipeline. In nature, they are made by a group of bacteria known as actinomycetes.

Pharmaceutical Technology
OCTOBER 31, 2022
The European Medicines Agency (EMA) has validated AbbVie ’s marketing authorization application (MAA) for epcoritamab (DuoBody-CD3xCD20) to treat relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) in adult patients following two or more lines of systemic therapy.

Pharmaceutical Technology
JUNE 2, 2023
Switzerland-based company Lonza has boosted its antibody-drug conjugates (ADCs) offering with the acquisition of Dutch biotechnology company Synaffix for a total consideration of $172m (€160m). The deal includes $107.17m (€100m) of initial financial consideration in cash and an additional $64.3m (€60m) in performance-based consideration.

pharmaphorum
MAY 13, 2021
The UK government has got cold feet over plans to buy a million doses of AstraZeneca’s antibody treatment for coronavirus, according to a press report. Bloomberg quoted two sources close to the matter saying that the government is rethinking the plans as AZ prepares to announce late-stage data for the antibody called AZD7442.

BioPharma Reporter
AUGUST 1, 2023
Renaissance Pharma, a company focused on the development of life changing therapies in pediatric rare disease, has entered into an exclusive license agreement with St. Jude Childrenâs Research Hospital for Hu14.18, a humanised antibody in development by the hospital for the treatment of newly diagnosed high-risk neuroblastoma.

The Pharma Data
AUGUST 20, 2020
Poor regulation of antibodies tests – that could indicate if someone has had coronavirus – could be putting the public at risk, doctors have warned. It is still not known whether having antibodies will protect people from a second infection. Coronavirus infection triggers the immune system to produce antibodies.

XTalks
OCTOBER 22, 2024
Vyloy is a first-in-class monoclonal antibody that targets the CLDN18.2 The FDA had rejected Vyloy in January due to “unresolved deficiencies following its pre-license inspection of a third-party manufacturing facility” for the drug. targeting antibody-drug conjugate. Astellas acquired Vyloy through its $1.4
Pharmaceutical Technology
AUGUST 25, 2022
The European Commission (EC) has granted conditional marketing authorisation (CMA) for BioMarin Pharmaceutical ’s gene therapy, Roctavian (valoctocogene roxaparvovec), to treat adults with severe haemophilia A (congenital Factor VIII deficiency).

Delveinsight
DECEMBER 8, 2020
KaliVir, Astellas Pharma forms a licensing deal for VET2-L2 oncolytic virus. KaliVir Immunotherapeutics and Astellas Pharma entered into a worldwide exclusive licensing agreement for the development, research, and commercialization of VET2-L2 to widen the horizon of therapeutic approaches available in the Immuno-Oncology market.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?



Let's personalize your content