Ipsen licenses ‘cutting-edge’ ADC from Sutro Biopharma in $900m deal
BioPharma Reporter
APRIL 2, 2024
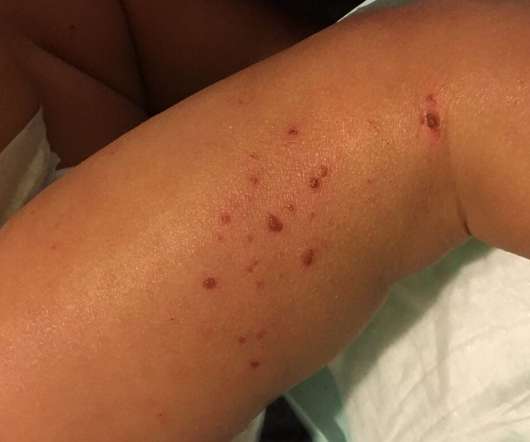
Ipsen is expanding its oncology pipeline by securing the global licensing rights to an antibody-drug conjugate (ADC) for solid tumors from Sutro Biopharma, its first drug in the category.













































Let's personalize your content