Bone Void Filler Cerament G: The Combination Product by BONESUPPORT Gets Market Authorization from the FDA
XTalks
MAY 25, 2022
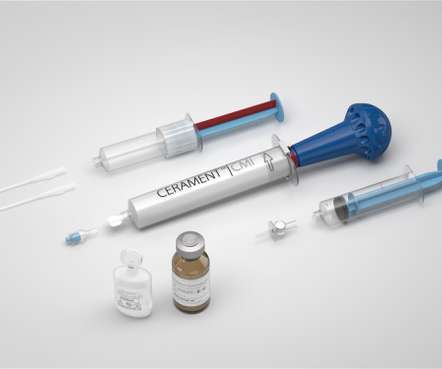
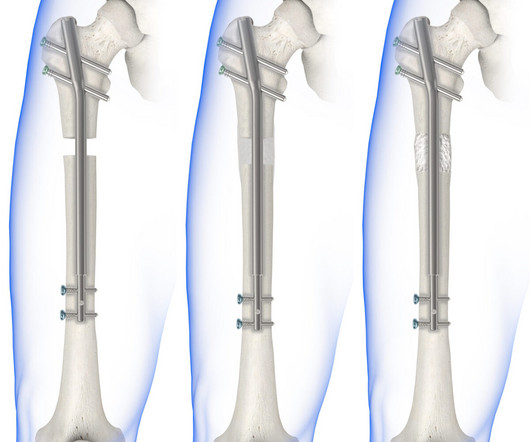
Ceramanet G, a bone void filler with the antibiotic gentamicin, fills bone voids and immediately begins bone healing with protection. Cerament G is a bone void filler treatment that can fill the gaps in the affected bones and simultaneously deliver the antibiotic gentamicin to reduce the risk of reinfection. mg/mL of cerament paste.



























Let's personalize your content