FDA approves Ascendis drug for rare endocrine condition
Bio Pharma Dive
AUGUST 12, 2024
The clearance of Yorvipath for hypoparathyroidism was some time coming for Ascendis, which had resubmitted after receiving a rejection last year.

Bio Pharma Dive
AUGUST 12, 2024
The clearance of Yorvipath for hypoparathyroidism was some time coming for Ascendis, which had resubmitted after receiving a rejection last year.
Pharmaceutical Technology
AUGUST 12, 2024
As antibody drug conjugate (ADC)-centred deals dominate the oncology space, biotechs are using newer targets and linkers to differentiate themselves.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
AUGUST 9, 2024
The decision to turn down an application from Lykos Therapeutics comes at a pivotal time for psychedelics research, which, after decades of dismissal, has recently gained momentum.

AuroBlog - Aurous Healthcare Clinical Trials blog
AUGUST 8, 2024
Last week, the Australian Therapeutic Goods Administration added intravenous (IV) fluids to the growing list of medicines in short supply. The shortage is due to higher-than-expected demand and manufacturing issues. Two particular IV fluids are affected: saline and compound sodium lactate (also called Hartmann’s solution). Both fluids are made with salts.

Rethinking Clinical Trials
AUGUST 7, 2024
In this Friday’s PCT Grand Rounds, Greg Simon and Susan Shortreed of the Kaiser Permanente Washington Health Research Institute (KPWHRI) will present “Does Starting Buprenorphine Prevent Suicidal Behavior? What Trial Should We Emulate?” The Grand Rounds session will be held on Friday, August 9, 2024, at 1:00 pm eastern. Simon is a senior investigator at KPWHRI and a research professor of psychiatry and behavioral sciences at the University of Washington.
Worldwide Clinical Trials
AUGUST 9, 2024
Authors: Matt Cooper, PhD, Executive Director, Therapeutic Strategy Lead, Oncology; Megan Morrison, Vice President, Asia Pacific Strategy Lead Adaptive trial designs have become essential in oncology, offering a flexible and efficient approach for conducting clinical trials. By allowing protocol adjustments based on interim data analysis, these designs preserve the integrity of studies while effectively addressing the unique challenges of multi-regional clinical trials (MRCTs).
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Pharmaceutical Technology
AUGUST 8, 2024
Coming in response to a rise in teenage overdoses across the US, the Purdue auto-injector device is designed to be used by anyone.
AuroBlog - Aurous Healthcare Clinical Trials blog
AUGUST 8, 2024
The Indian healthcare sector is now concerned on quality and accuracy of Point-of-Care (PoC) diagnostic test results. This apprehension is because of limited training or experience with these devices and those handling the same. Hands-on practice and ongoing education to remain updated with changes in technology are issues.

Rethinking Clinical Trials
AUGUST 8, 2024
Speaking at the NIH Pragmatic Trials Collaboratory’s 2024 Annual Steering Committee Meeting in May, Commissioner of Food and Drugs Robert Califf and NIH Director Monica Bertagnolli discussed the intersection of pragmatic research and national health priorities. Califf and Bertagnolli spoke about the role the NIH Collaboratory can play in promoting scientifically rigorous, pragmatic clinical research that addresses the urgent need for better and faster implementation of research findings into pat

Fierce Pharma
AUGUST 12, 2024
Ascendis Pharma should be well prepared for the U.S. | After two delays, the FDA has finally signed off on Ascendis Pharma's hormone replacement therapy Yorvipath, also known as TransCon PTH, which is the first approved product for hypoparathyroidism in adults in the U.S.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations
Pharmaceutical Technology
AUGUST 8, 2024
Novartis has secured US Food and Drug Administration (FDA) accelerated approval for Fabhalta (iptacopan) to treat igAN.
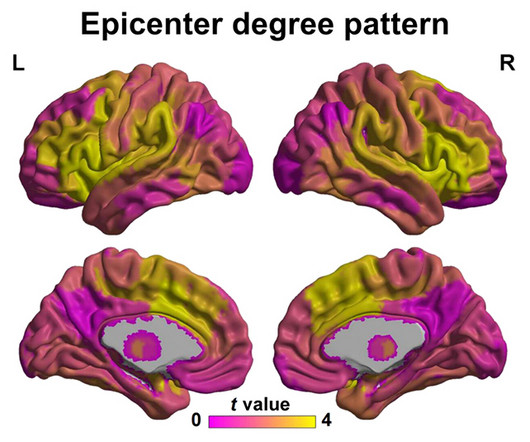
AuroBlog - Aurous Healthcare Clinical Trials blog
AUGUST 7, 2024
Scientists think they may have found locations in the brain where schizophrenia first emerges, potentially making the neurological disorder’s onset easier to diagnose from a standard MRI brain scan.
pharmaphorum
AUGUST 7, 2024
Explore the innovative applications of mRNA technologies beyond COVID-19 vaccines. Learn about the potential and future developments in this groundbreaking field.

Fierce Pharma
AUGUST 7, 2024
The first horse carrying Novartis’ immunoglobulin A nephropathy (IgAN) troika has crossed the FDA finish line. | The first horse carrying Novartis’ immunoglobulin A nephropathy (IgAN) troika has crossed the FDA finish line.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Bio Pharma Dive
AUGUST 8, 2024
Sales of the in-demand obesity drug crested $1 billion in the second quarter as improved Lilly production helped the company fulfill wholesaler backorders.

Pharmaceutical Technology
AUGUST 9, 2024
Days after competitor Novo Nordisk posted underwhelming Wegovy sales, Eli Lilly cited “strong performance” for Zepbound and Mounjaro.

AuroBlog - Aurous Healthcare Clinical Trials blog
AUGUST 7, 2024
India continues to face significant challenges in boosting its organ donation rates, currently languishing at just over one per million population. Despite widespread public support, the country struggles to increase its organ donation rates, due in part to a combination of legal misconceptions, lack of awareness within the medical community, and cultural barriers.

pharmaphorum
AUGUST 9, 2024
Eli Lilly's oral tau drug fails to show efficacy in a phase 2 trial in Alzheimer's disease in another setback for the class

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.
Fierce Pharma
AUGUST 9, 2024
As Purdue Pharma attempts to restore its image following its role in | As Purdue Pharma attempts to restore its image following its role in the U.S. opioid crisis, the Connecticut-based company has scored a win with a green light for a new overdose rescue treatment.

Bio Pharma Dive
AUGUST 12, 2024
Results from a Phase 3 study could help Pfizer broaden use of its vaccine Abrysvo to younger adults whose medical history puts them at higher risk.

Pharmaceutical Technology
AUGUST 8, 2024
The US Food and Drug Administration (FDA) has approved Amneal Pharmaceuticals' CREXONT extended-release capsules for PD.

Velocity Clinical Research
AUGUST 8, 2024
It’s been just over a year since the monoclonal antibody Lecanemab received traditional approval from the FDA as a treatment for Alzheimer’s disease. This marked a turning point in the disease’s treatment, a drug that interrupted progression for the first time rather than simply addressing the symptoms. It was this type of breakthrough that Robert Cupelo, MD hoped for when he joined Velocity’s Syracuse site as Principal Investigator in 2017.

Advertiser: FourKites
A research study conducted by The Journal of Commerce and FourKites surveyed hundreds of international shippers, exploring how their usage of global supply chain visibility technology has evolved since the onset of global disruptions caused by COVID-19. For international shippers, ocean freight visibility has evolved from optional to essential and satisfaction with visibility varies greatly depending on how it is obtained and delivered.

pharmaphorum
AUGUST 12, 2024
Three controversial decisions by NICE on NHS use of new multiple myeloma therapies are heading for the end of comment and appeals processes.

Fierce Pharma
AUGUST 9, 2024
With Gilead's stated focus to grow in oncology, the company's recent quarterly updates have placed a heavy emphasis on its developments in the cancer space. | The HIV-focused drugmaker looks to position its long-acting Sunlenca as a prime PrEP option after the med aced a phase 3 study.

Bio Pharma Dive
AUGUST 9, 2024
The deal hands Merck a cancer medicine already in human testing, but that the company sees as a potential treatment for autoimmune conditions, too.

Pharmaceutical Technology
AUGUST 8, 2024
Conduit plans to advance two assets, AZD1656 and AZD5658, into Phase II testing for autoimmune disorders.
XTalks
AUGUST 12, 2024
Mendel AI and the University of Massachusetts Amherst (UMass Amherst) have published key data on addressing the critical issue of hallucinations in AI-generated medical summaries using a pioneering artificial intelligence (AI) framework called “Hypercube”. This collaboration is a step forward in ensuring the reliability and safety of AI applications in healthcare.
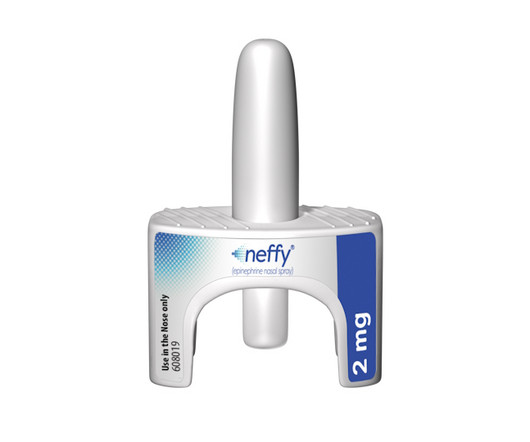
pharmaphorum
AUGUST 12, 2024
ARS Pharma gets FDA approval for epinephrine nasal spray neffy, the first needle-free alternative to EpiPen-style autoinjectors for serious allergic reactions.

Fierce Pharma
AUGUST 8, 2024
While a glut of drugmakers have raised their 2024 financial guidance after the second quarter, those upgraded sales expectations pale in comparison to Eli Lilly’s new forecast for the year. | Lilly, which reported a 36% sales increase year-over-year to $11.3 billion in the second quarter, is raising its guidance for the full year by a staggering $3 billion.

Bio Pharma Dive
AUGUST 7, 2024
Executives at Amgen had few updates on their drug MariTide, but defended what they see as the once-monthly shot’s competitive profile.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.
Let's personalize your content