Streamlining mesenchymal stem cell (MSC) expansion
Pharmaceutical Technology
JANUARY 21, 2025
With increasing demand for cell and gene therapies, mesenchymal stem cells (MSCs) have gained attention for their versatility and potential.

Pharmaceutical Technology
JANUARY 21, 2025
With increasing demand for cell and gene therapies, mesenchymal stem cells (MSCs) have gained attention for their versatility and potential.

Bio Pharma Dive
JANUARY 17, 2025
Novo sells semaglutide as Ozempic and Rybelsus for diabetes and as Wegovy for obesity. In price talks, CMS will treat the different forms as a single product.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
JANUARY 16, 2025
Pharma dealmaking for drugs invented in China is putting pressure on U.S. biotechs to compete harder, according to investors and executives interviewed by BioPharma Dive at the J.P. Morgan Healthcare Conference.

Rethinking Clinical Trials
JANUARY 21, 2025
Dr. Corita Grudzen and Dr. Keith Goldfeld, principal investigators for PRIM-ER An evidence-based training program to improve the capacity of emergency department care teams to communicate with seriously ill older patients about palliative care did not lead to lower rates of hospital admission, according to the results of the PRIM-ER trial. The results were published online ahead of print in JAMA.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Worldwide Clinical Trials
JANUARY 21, 2025
Jayaprakash Kotha, MBBS, PhD, ASCP (SH), Vice President, Bioanalytical Laboratory Satish Kumar, MBB, Head of Process Improvement Continuous Innovation is a Cornerstone of Bioanalysis Approximately 80% of drugs that begin the research process fail to reach approval. What is one contributing factor that sets the 20% that do apart from the rest? Rigorous procedures to ensure that drugs are effective and safe.
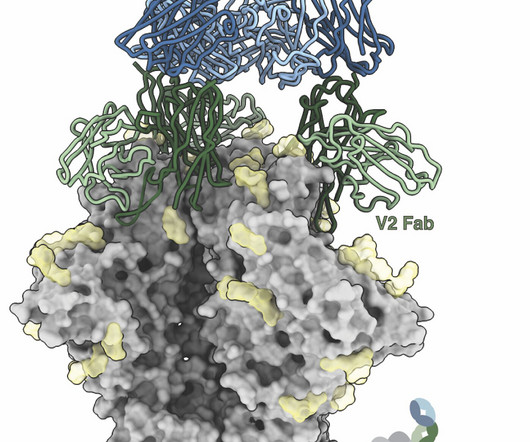
Medical Xpress
JANUARY 17, 2025
Many vaccines work by introducing a protein to the body that resembles part of a virus. Ideally, the immune system will produce long-lasting antibodies recognizing that specific virus, thereby providing protection.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Pharmaceutical Technology
JANUARY 16, 2025
Six out of 31 total new small molecules approved by the FDA in 2024 were produced by Wuxi, said the company's co-CEO.

Rethinking Clinical Trials
JANUARY 15, 2025
Dr. Christopher Lindsell In this Friday’s PCT Grand Rounds, Chris Lindsell of Duke University will present “Design for Diversity.” The Grand Rounds session will be held on Friday, January 17, 2025, at 1:00 pm eastern. Lindsell is professor and cochief of biostatistics and bioinformatics, director of data science and biostatistics at the Duke Clinical Research Institute, and director of biostatistics and bioinformatics at the Duke Clinical and Translational Science Instituteall

Pharma Times
JANUARY 15, 2025
Funds to accelerate commercialisation and clinical trials of diagnostic tests

XTalks
JANUARY 17, 2025
Johnson & Johnson (J&J) has announced its $14.6 billion acquisition of Intra-Cellular Therapies, which brings Caplyta (lumateperone) to its growing portfolio of mental health treatments. The deal also includes a cash payment of $132 per share for all outstanding shares of Intra-Cellular Therapies. Caplyta is an oral medication that was first approved in 2019 for the treatment of schizophrenia in adults, followed by a 2021 approval for depressive episodes associated with bipolar I and II

Bio Pharma Dive
JANUARY 16, 2025
Roche's core research spans five large areas. Boris Zaïtra, who now leads corporate business development, is confident his team of dealmakers can juggle them.
Pharmaceutical Technology
JANUARY 16, 2025
Eli Lilly has received approval from the US FDA for Omvoh to treat moderately to severely active Crohn's disease in the adult population.
pharmaphorum
JANUARY 17, 2025
Hospitals should expand and speed up testing of people hospitalised with influenza to see if they are suffering from bird flu, says CDC.

Rethinking Clinical Trials
JANUARY 16, 2025
Speakers Dana Dailey PT, PhD Assistant Research Scientist Physical Therapy and Rehabilitation Science University of Iowa Associate Professor, Physical Therapy Department St. Ambrose University Heather Schacht Reisinger, PhD Director, Implementation Science Center Associate Director for Engagement, Integration, and Implementation Institute for Clinical and Translational Science Professor, Division of General Internal Medicine at the University of Iowa Slides Keywords Commun

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

XTalks
JANUARY 16, 2025
BlueRock Therapeutics, a subsidiary of Bayer AG, is making waves in the treatment of Parkinsons disease by advancing its investigational cell therapy, bemdaneprocel, to a Phase III clinical trial. Called exPDite-2, the registrational trial meaning a trial designed to gather the necessary data for potential regulatory approval is slated to begin in the first half of 2025.

Pharmaceutical Technology
JANUARY 16, 2025
At the JP Morgan Conference 2025, CEO Chen said the potential of the Biosecure Act passing has had only a minimal chilling effect on clients.
Pharma Times
JANUARY 15, 2025
Promising results from mouse study lead to expanded trials

Medical Xpress
JANUARY 18, 2025
Every 40 seconds, someone in the United States has a stroke. For survivors of the most common type of stroke, called an ischemic stroke, only about 5 percent fully recover. Most others suffer from long-term problems, including weakness, chronic pain, or epilepsy.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

XTalks
JANUARY 16, 2025
Qiagen received FDA clearance for its new QIAstat-Dx mini gastrointestinal panel last week. According to Qiagen, the test is the first in a series of QIAstat-Dx gastrointestinal (GI) panel tests for clinical use. The new QIAstat-Dx Gastrointestinal Panel 2 mini B&V (bacterial and viral) is designed for the rapid outpatient diagnosis of gastrointestinal conditions.

Bio Pharma Dive
JANUARY 21, 2025
Moderna has been testing an mRNA candidate for influenza viruses like the H5 and H7 strains that are seen as pandemic threats. Others, including GSK and Pfizer, are also at work on similar shots.

Pharmaceutical Technology
JANUARY 17, 2025
Shionogis S-892216 is being developed as a pre-exposure prophylaxis (PrEP) drug for Covid-19. Credit: seksan Mongkhonkhamsao via Getty Images.

pharmaphorum
JANUARY 19, 2025
Sun Pharma's Taro unit has made a bid to acquire Canadian biotech Antibe, a developer of drugs for pain and inflammation

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.
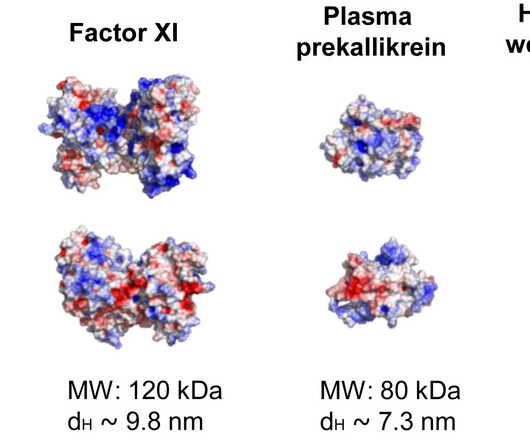
Medical Xpress
JANUARY 17, 2025
Researchers at Oregon Health & Science University have uncovered how a molecule found on certain bacteria may drive blood clotting in sepsis, a life-threatening condition that causes about 8 million deaths per year.
XTalks
JANUARY 15, 2025
Known for its AI-driven diagnostic tools, IMVARIA, a Berkeley-based health tech company, recently earned the FDAs 510(k) clearance of ScreenDx, a first-of-its-kind algorithm designed to identify potential interstitial lung disease (ILD) cases. This follows their earlier success with Fibresolve , a tool for idiopathic pulmonary fibrosis (IPF) that became the first FDA Breakthrough-designated AI diagnostic for lung fibrosis.

Bio Pharma Dive
JANUARY 15, 2025
The review, which was spurred by controversy over the FDA's Aduhelm approval, recommends two main changes in agency procedures.

Pharmaceutical Technology
JANUARY 20, 2025
Moderna is set to receive $590m in funding from the HHS to expedite the development of mRNA-based pandemic influenza vaccines.

Advertiser: FourKites
A research study conducted by The Journal of Commerce and FourKites surveyed hundreds of international shippers, exploring how their usage of global supply chain visibility technology has evolved since the onset of global disruptions caused by COVID-19. For international shippers, ocean freight visibility has evolved from optional to essential and satisfaction with visibility varies greatly depending on how it is obtained and delivered.

Pharma Times
JANUARY 20, 2025
Collaboration aims to enhance accessibility of medicines information

Antidote
JANUARY 16, 2025
When preparing for flu season, those with asthma may have concerns about how the virus can affect their respiratory health and breathing. While asthma is a manageable condition, it does require extra care when dealing with seasonal illnesses like the flu. In this blog, we'll explore how asthma and the flu interact, provide tips for managing both, and offer resources to help you stay informed and prepared.

pharmaphorum
JANUARY 15, 2025
Adverse events have led Keros to abandon a phase 2 trial of pulmonary arterial hypertension drug cibotacept, a would-be rival to MSD's Winrevair

Bio Pharma Dive
JANUARY 15, 2025
While Biogen has made an opportunistic bid for partner Sage, its top executive seemed to play down the urgency for his company to go after larger deals.
Let's personalize your content