Food Allergy Awareness Week 2024: How can new treatments target an unmet need?
Pharmaceutical Technology
MAY 16, 2024
IgGenix is planning to initiate a clinical trial later this year investigating its peanut allergy monoclonal antibody IGXN001.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
MAY 16, 2024
IgGenix is planning to initiate a clinical trial later this year investigating its peanut allergy monoclonal antibody IGXN001.

XTalks
MARCH 10, 2025
Originally approved by the FDA over two decades ago for the treatment of moderate to severe persistent asthma, Xolair recently also gained approval for managing food allergies. Omalizumab is an anti-IgE antibody that works by blocking the effects of IgE, a key driver in many allergic reactions. billion by 2030.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Scienmag
APRIL 21, 2021
Credit: NIAID A Phase 2/3 trial to evaluate a new fully-human polyclonal antibody therapeutic targeted to SARS-CoV-2, called SAB-185, has begun enrolling non-hospitalized people with mild or moderate cases of COVID-19.

Pharmaceutical Technology
JULY 18, 2022
According to positive interim data from the Phase II/III KidCOVE clinical trial, the vaccine offered a strong neutralising antibody response in children of the age group of six months to five years following administration of the two-dose initial regimen of the mRNA-1273 vaccine.

Medical Xpress
DECEMBER 22, 2022
A weekly dose of dupilumab, a monoclonal antibody, led to a reduction of symptoms and tissue improvement in young adults and adolescents with eosinophilic esophagitis (EoE), according to a new study published in the New England Journal of Medicine.

Pharmaceutical Technology
JANUARY 8, 2023
In 2023, the pharmaceutical industry will mark 20 years since Xolair, an anti-IgE antibody, became the first biologic approved to treat asthma. Since then, the US FDA, EMA, and other agencies have approved several biologic antibodies targeting the inflammatory cytokines IL-4, IL-13, IL-5, and others for asthma. Ongoing efforts.

The Pharma Data
OCTOBER 27, 2020
The NIH’s National Institute of Allergy and Infectious Diseases (NIAID) has halted a clinical trial evaluating Eli Lilly’s investigational monoclonal antibody, LY-CoV555 (bamlanivimab), in combination with remdesivir for the treatment of COVID-19 patients. Source link.

Medical Xpress
MARCH 6, 2023
A clinical trial testing a freeze-dried, temperature-stable experimental tuberculosis (TB) vaccine in healthy adults found that it was safe and stimulated both antibodies and responses from the cellular arm of the immune system. A non-temperature stable form of the candidate previously had been tested in several clinical trials.

Pharmaceutical Technology
AUGUST 5, 2022
According to GlobalData’s Pharma Intelligence Center, there are only six drugs in active development for CJD, of which only one has been in clinical trials. There are only a few antibodies that can specifically recognize the (abnormal) version of the prion protein.

pharmaphorum
AUGUST 3, 2020
While the world waits an effective coronavirus vaccine, Eli Lilly has started late-stage human testing an antibody drug as an alternative way to prevent viral transmission in high-risk locations. Staff will also be deployed to minimise the impact of the trial on facilities that don’t normally run clinical trials.

The Pharma Data
AUGUST 7, 2020
The governing body claims this proposal could have positive effects on mitigating burdensome requirements for gene therapy clinical trials in the future. In this light, Pharma IQ’s weekly round-up focuses on advancing therapies and clinical trials to combat Covid-19.
The Pharma Data
APRIL 3, 2021
The CoVIg-19 Plasma Alliance today announced that the Phase 3 Inpatient Treatment with Anti-Coronavirus Immunoglobulin (ITAC) clinical trial sponsored and funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), did not meet its endpoints. About the ITAC Trial.
Pharmaceutical Technology
JUNE 8, 2023
With respect to immunogenicity, neutralising antibody titers against ancestral Spike and variants of concern were boosted by the samRNA candidate, and contrasting to authorised vaccines, persisted through at least 6 months following the booster dose. of these are in the pre-clinical stage.

The Pharma Data
NOVEMBER 16, 2020
and the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. As part of the arrangement under OWS, representatives from NIAID, BARDA and Moderna are part of the oversight group that receives recommendations from the trial’s independent DSMB. El Sahly, M.D., El Sahly, M.D.,

Scienmag
AUGUST 11, 2020
Baylor Scott & White Research Institute becomes first site in the world to conduct new trial testing experimental monoclonal antibodies as a treatment for hospitalized COVID-19 patients Credit: Baylor Scott & White Research Institute DALLAS, Texas – On Wednesday, August 5, in Dallas, just one day after the initiative was launched by (..)

The Pharma Data
OCTOBER 26, 2020
The NIH’s National Institute of Allergy and Infectious Diseases (NIAID) has halted a clinical trial evaluating Eli Lilly’s investigational monoclonal antibody, LY-CoV555 (bamlanivimab), in combination with remdesivir for the treatment of COVID-19 patients.

Delveinsight
AUGUST 6, 2020
Zydus starts phase 2 COVID-19 vaccine trial after clearing safety test. Zydus Cadila has finished a phase 1 clinical trial of its COVID-19 vaccine ZyCoV-D, setting it up to move straight into a 1,000-subject phase 2 study. FDA turns back peanut allergy patch of DBV Technologies. The agency raised no safety concerns.

Scienmag
MAY 27, 2021
New Penn Medicine study shows how T cells compensate when other immune cells go down PHILADELPHIA–Antibodies aren’t the only immune cells needed to fight off COVID-19 — T cells are equally important and can step up to do the job when antibodies are depleted, suggests a new Penn Medicine study of blood cancer patients with […]. (..)

The Pharma Data
APRIL 11, 2021
Basel, 12 April 2021 – Roche (SIX: RO, ROG; OTCQX: RHHBY) today confirmed positive results from the phase III REGN-COV 2069 trial assessing the ability of the investigational antibody cocktail casirivimab and imdevimab to reduce the risk and burden of COVID-19 infection among household contacts of SARS-CoV-2 infected individuals.

XTalks
NOVEMBER 30, 2023
The safety and efficacy of Cabenuva were established through two randomized, open-label, controlled clinical trials involving 1,182 HIV-infected adults who were virologically suppressed (HIV-1 RNA less than 50 copies/mL) before initiating Cabenuva treatment. The Phase I trial for HB-500 is scheduled to start in the first half of 2024.

Scienmag
MARCH 31, 2021
New findings from a Michigan Medicine study reveal that antibody testing is predictive of prior COVID-19 infection, and rapid screening methods – even from finger pricks – are effective testing tools. Researchers analyzed antibody tests conducted on more than 500 subjects […].

The Pharma Data
NOVEMBER 9, 2020
Food and Drug Administration (FDA) today granted Emergency Use Authorization (EUA) for Eli Lilly and Company’s (NYSE: LLY) investigational neutralizing antibody bamlanivimab (LY-CoV555) 700 mg. Important Safety Information There are limited clinical data available for bamlanivimab. INDIANAPOLIS, Nov.

The Pharma Data
JANUARY 26, 2021
. “These exciting results, which replicate positive Phase 2 data in a much larger set of patients, add valuable clinical evidence about the role neutralizing antibodies can play in fighting this pandemic. Important Safety Information There are limited clinical data available for bamlanivimab.

Delveinsight
JANUARY 21, 2021
Eli Lilly, via its Loxo Oncology biotech unit, is enlisting to a three-therapy pact with Merus concentrated on T-cell redirecting bispecific antibody work. These will come out of Merus’ so-called Biclonics platform that develops CD3-engaging, T-cell redirecting bispecific antibody therapies. billion in total for three drugs.

XTalks
JANUARY 20, 2021
However, IBS patients do not have allergies to any given foods, nor any gastrointestinal conditions, such as celiac disease. Ovalbumin led to increased dietary-antigen-specific IgE antibodies in the mice that were limited to the intestine. Moreover, the response did not induce symptoms typical of a food allergy.

The Pharma Data
OCTOBER 26, 2020
Diagnostics Update : To-date the FDA has authorized 284 individual EUAs, which include 221 molecular tests, 56 antibody tests and 7 antigen tests. . Only a fraction of intravenous antibody treatments will make their way to the lungs of COVID-19 patients, which is where the infection is primarily located. Please read more here. .
XTalks
JANUARY 27, 2021
The company used in vitro neutralization antibody studies to evaluate the activity of Moderna’s vaccine-generated antibodies in human sera against several variants of the novel coronavirus, including the B.1.1.7 The in vitro study evaluated the ability of mRNA-1273-generated antibodies to neutralize the new SARS-CoV-2 variants.

Delveinsight
AUGUST 27, 2020
The phase 1 study that is being run by the National Institute of Allergy and Infectious Diseases tests three dose levels of the vaccine, mRNA-1273, given in two injections a month apart in 120 adults. Freenome secures USD 270 Million to boost its colorectal cancer blood test.

Scienmag
MAY 13, 2021
New study suggests COVID vaccines may still be helpful for patients with antibody deficiency disorders According to data from a cohort of adult and pediatric patients with antibody deficiencies, patients that often fail to make protective immune responses to infections and vaccinations showed robust T-cell activity and humoral immunity against SARS-CoV-2 (..)

Pfizer
JULY 8, 2022
Favorable safety profile observed across more than 2,200 adolescents who participated in the clinical trial. Today’s approval is based on data from a Phase 3 clinical trial of 2,260 participants 12 through 15 years of age. NEW YORK and MAINZ, GERMANY, July 8, 2022 — Pfizer Inc. have completed a primary series.
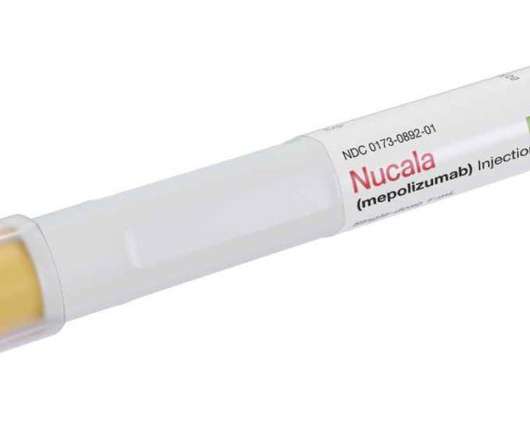
The Pharma Data
JULY 29, 2021
today announced that the US Food and Drug Administration (FDA) has approved Nucala (mepolizumab), a monoclonal antibody that targets interleukin-5 (IL-5), as a treatment for patients with chronic rhinosinusitis with nasal polyps (CRSwNP). With 41 clinical trials, mepolizumab has been studied in over 4,000 patients.

Pfizer
JULY 26, 2022
Tell your vaccination provider about all of the vaccine recipient’s medical conditions, including if the vaccine recipient: has any allergies. The Pfizer-BioNTech COVID-19 Vaccine authorized for use in children 6 months through 11 years of age should not be used interchangeably with COMIRNATY® (COVID-19 Vaccine, mRNA). has a fever.
The Pharma Data
JANUARY 24, 2021
With a presidential inauguration and a federal holiday, it wasn’t an enormously busy week for clinical trial news, but there was a fair amount, nonetheless. It inked a deal with the National Institute of Allergy and Infectious Diseases (NIAID) to launch a Phase I trial. Read on to see. COVID-19-Related.

The Pharma Data
NOVEMBER 25, 2020
FDA’s approval of remdesivir (Veklury) was supported by the agency’s independent, in-depth analysis of data from three randomized, controlled clinical trials that included patients hospitalized with mild-to-severe COVID-19. Researchers evaluated the clinical status of subjects on Day 14. The design of ACTT-1 (i.e.,

Pfizer
SEPTEMBER 28, 2022
Its broad portfolio of oncology product candidates includes individualized and off-the-shelf mRNA-based therapies, innovative chimeric antigen receptor T cells, bispecific immune checkpoint modulators, targeted cancer antibodies and small molecules. Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5),

The Pharma Data
MAY 22, 2023
These results will also be presented in the “Breaking News: Clinical Trial Results in Pulmonary Medicine” session on May 22. “I’ve MSPH, Associate Professor at the University of Alabama at Birmingham Division of Pulmonary, Allergy, and Critical Care Medicine, and a co-principal investigator of the trial. “I’ve

XTalks
OCTOBER 26, 2023
Some of the vaccines are already in clinical trials, including a universal influenza vaccine developed by researchers at the National Institute of Allergy and Infectious Diseases’ (NIAID) Vaccine Research Center (VRC), part of the National Institutes of Health (NIH). “A For COVID, it is much the same case.
The Pharma Data
JULY 15, 2021
Antibody and T-cell immune responses strong and stable at eight months after immunization Demonstrated neutralizing antibody activity against the Delta variant (B.1.617.2) Mature B-cells produce antibodies, which can help fight the virus that causes COVID-19. Data from the study conducted in collaboration with Dan Barouch, M.D.,

Pfizer
JULY 19, 2022
Tell your vaccination provider about all of the vaccine recipient’s medical conditions, including if the vaccine recipient: has any allergies. IMPORTANT SAFETY INFORMATION. has had myocarditis (inflammation of the heart muscle) or pericarditis (inflammation of the lining outside the heart). has a fever. has received another COVID-19 vaccine.

The Pharma Data
APRIL 14, 2021
government for its neutralizing antibody therapies authorized for emergency use as a treatment for COVID-19. Lilly’s bamlanivimab was the first neutralizing monoclonal antibody to receive emergency use authorization from the U.S. Eli Lilly and Company (NYSE: LLY) today announced changes to the purchase agreements with the U.S.

The Pharma Data
JULY 6, 2021
This adds to the robust body of clinical data supporting our single-shot vaccine’s ability to protect against multiple variants of concern.”. Demonstrated strong neutralizing antibody activity against the Delta (B.1.617.2) Global Head, Janssen Research & Development, Johnson & Johnson. 1.351), the Gamma (P.1) 1.429), Kappa (B.1.617.1)

The Pharma Data
MARCH 4, 2021
Adverse events in the Phase 3 trial that were more commonly observed with Dupixent included injection site reactions, viral upper respiratory tract infections and eosinophilia. Detailed results from this Phase 3 trial will be published later this year. Dupilumab development program.

Bioengineer
JULY 23, 2021
“Given the respiratory tropism of the virus, it seems surprising that only seven of the nearly 100 SARS-CoV-2 vaccines currently in clinical trials are delivered intranasally,” Lund and Randall said. The seventh vaccine candidate is an inert protein subunit.

The Pharma Data
DECEMBER 23, 2020
Additionally, Phase 1 clinical data found INO-4800 to have a favorable safety and tolerability profile with no serious adverse events reported; only six Grade 1 adverse events (AEs) were observed, primarily minor injection site reactions. ” Findings from the Phase 1 Clinical Trial. mg and 2.0
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content