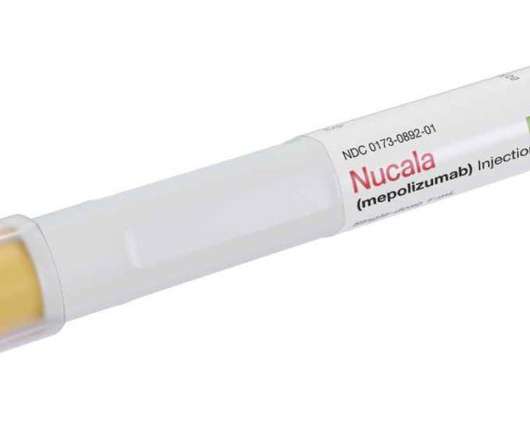
Top 5 Most Promising FDA New Drug Approvals Expected in the Second Half of 2024
XTalks
MAY 2, 2024
In 2023, the US Food and Drug Administration (FDA) approved a record-breaking 61 drugs, the most in history. Since January, the FDA has already signed off on more than a dozen novel drugs. The agency is reviewing applications for several highly anticipated drugs for approval in 2024.




































Let's personalize your content