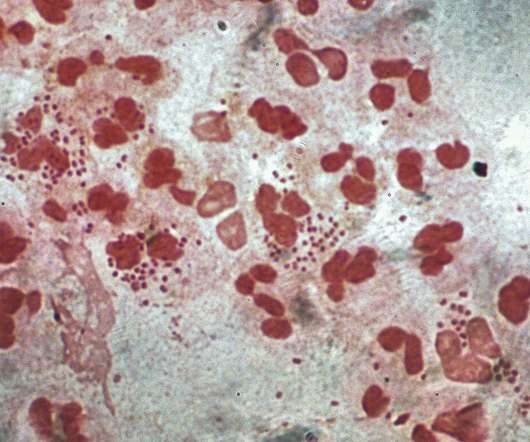
Intravacc gets NIAID contract for intranasal gonorrhoea vaccine development
Pharmaceutical Technology
OCTOBER 6, 2022
from the US National Institutes of Health (NIH) unit National Institute of Allergy and Infectious Diseases (NIAID) to develop a prophylactic intranasal vaccine against Neisseria gonorrhoeae (NG). Therapyx will develop and manufacture the IL-12-containing microspheres in the vaccine called GneX12.






















Let's personalize your content