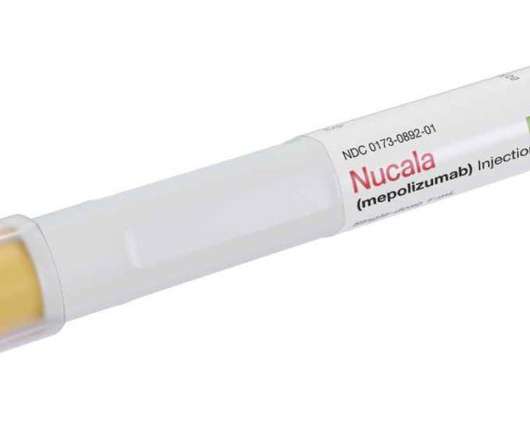
GSK announces FDA approval for Nucala (mepolizumab) for use in adults with chronic rhinosinusitis with nasal polyps
The Pharma Data
JULY 29, 2021
It is often characterised by raised eosinophil levels, in which soft tissue growth, known as nasal polyps, develop in the sinuses and nasal cavity. Mepolizumab is the first anti-IL-5 biologic to be approved for adult patients with CRSwNP in the US. It is not currently approved for use in COPD anywhere in the world.


























Let's personalize your content