First Single-Shot Vaccine in Fight Against Global Pandemic
The Pharma Data
MARCH 2, 2021
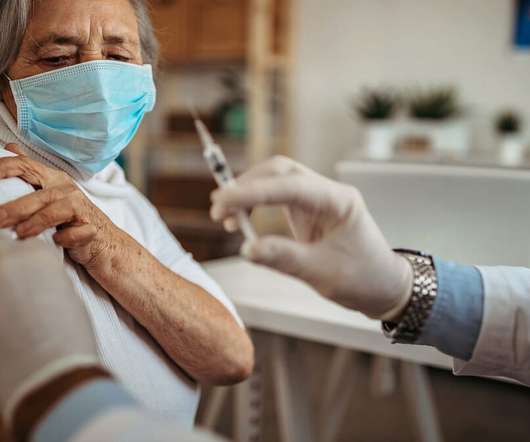
Food and Drug Administration (FDA) has issued Emergency Use Authorization (EUA) for its single-dose COVID-19 vaccine, developed by the Janssen Pharmaceutical Companies of Johnson & Johnson, to prevent COVID-19 in individuals 18 years of age and older. The terms of the EUA allow use of the vaccine while more data are gathered.


































Let's personalize your content