Evaxion and ExpreS²ion partner to develop new CMV vaccine
Pharmaceutical Technology
DECEMBER 6, 2022
In the partnership’s discovery phase, RAVEN, an artificial intelligence (AI) platform of Evaxion will be leveraged for designing a next-generation vaccine candidate that induces cellular as well as humoral/antibody responses. Using ExpreS 2 ion’s ExpreS2 platform, the company will manufacture the antigen constructs obtained from RAVEN.











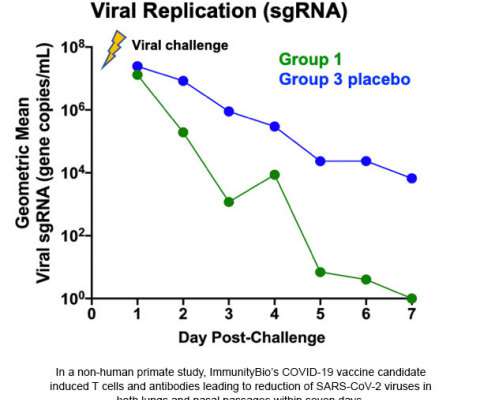




























Let's personalize your content