Trial to test monoclonal antibody for virus of growing concern
Drug Discovery World
JULY 2, 2024
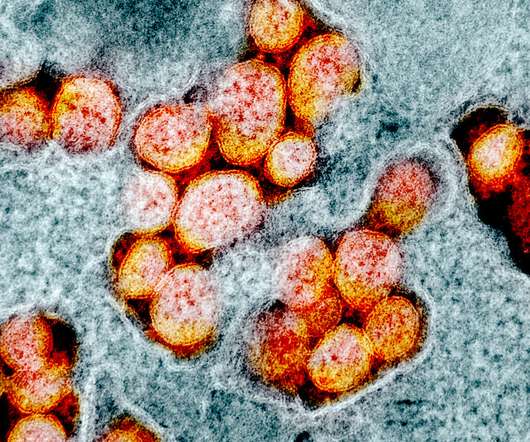
The National Institutes of Health (NIH) is sponsoring a clinical trial to evaluate the safety of an investigational monoclonal antibody to treat enterovirus D68 (EV-D68), which can cause severe respiratory and neurological diseases such as acute flaccid myelitis (AFM). Credit: NIAID and CDC.
































Let's personalize your content