Machine learning: A useful tool in the development of next generation antibody therapeutics
Drug Discovery World
MAY 21, 2024
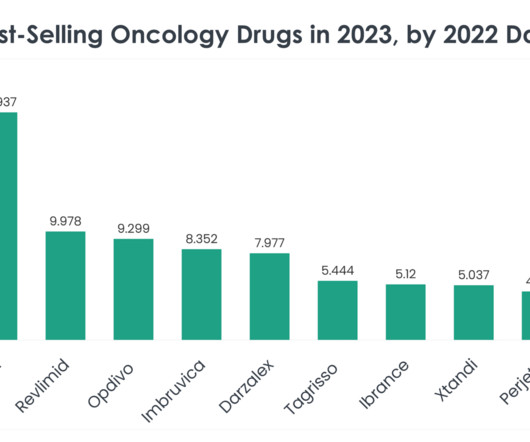
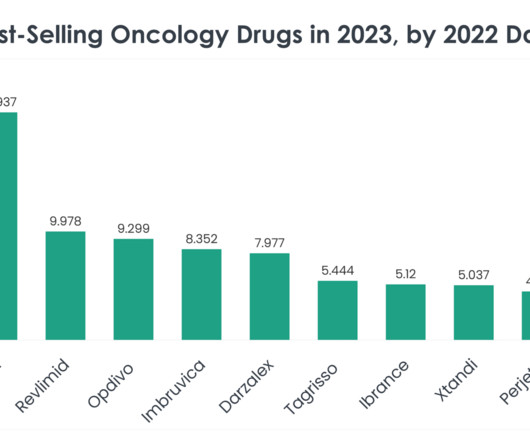
Ben Holland , CTO and Co-Founder of Antiverse discusses how artificial intelligence and machine learning are benefitting antibody discovery and design. Modern experimental procedures, such as immunisation, B-cell screening, and synthetic library generation, have been pivotal in developing approximately 80 FDA-approved antibody therapeutics.









































Let's personalize your content