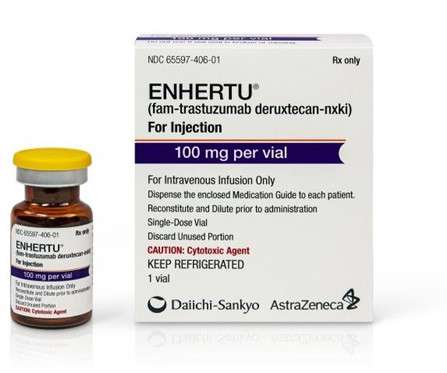
AstraZeneca-Daiichi Sankyo’s Enhertu gets US FDA approval for breast cancer
Pharmaceutical Technology
AUGUST 8, 2022
The approval was based on findings from the international, randomised, open-label Phase III DESTINY-Breast04 clinical trial. Furthermore, Enhertu’s safety profile was in line with prior clinical trials without any new safety concerns detected. Enhertu-treated subjects had a median overall survival (OS) of 23.4


































Let's personalize your content