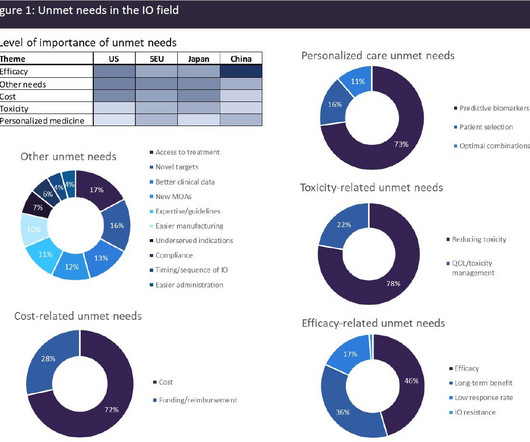
A broad range of unmet needs remains in the immuno-oncology space
Pharmaceutical Technology
MAY 2, 2023
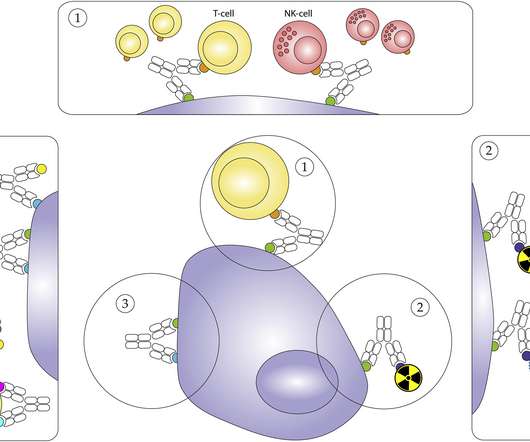
IO agents include the classes of immune checkpoint modulators, cell therapies, bispecific antibodies, oncolytic viruses, therapeutic vaccines, and cytokines. All currently approved CAR-Ts are autologous, with the patient’s T-cells being genetically engineered to target antigens expressed by the cancerous cells.
























Let's personalize your content