Biologic CAR T cell engager approved for UK clinical trial
Drug Discovery World
AUGUST 9, 2023
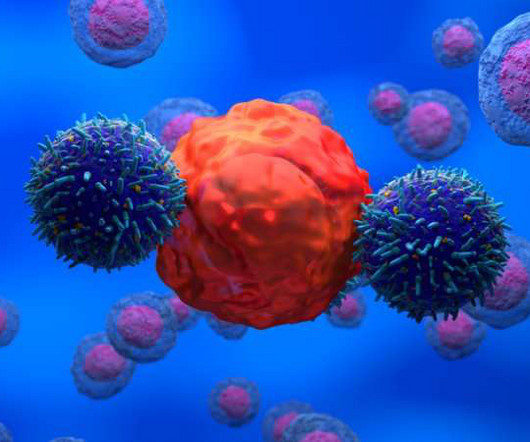
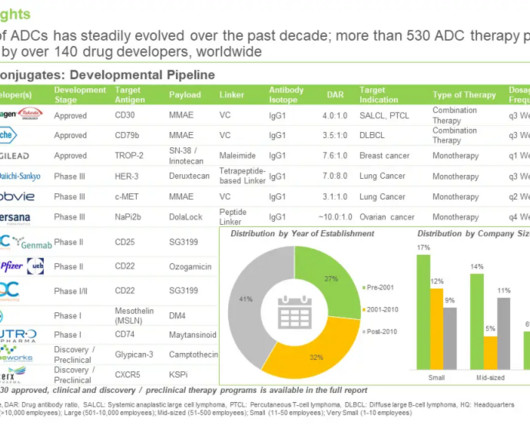
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has granted Aleta Biotherapeutics a clinical trial authorisation (CTA) to evaluate biologic ALETA-001 in a Phase I/II clinical trial in patients with B-cell malignancies who are relapsed/refractory to CD19 CAR T cell therapy.




































Let's personalize your content