MHRA authorises Moderna’s omicron-containing bivalent booster
Pharma Times
AUGUST 15, 2022
Study results show the candidate has demonstrated significantly higher antibodies against Omicron
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Pharma Times
AUGUST 15, 2022
Study results show the candidate has demonstrated significantly higher antibodies against Omicron
Pharmaceutical Technology
OCTOBER 19, 2022
Syncromune and Biocytogen Pharmaceuticals’ wholly owned subsidiary Eucure Biopharma have entered an exclusive global licence agreement for OX40 antibody YH002 and two other active ingredients. . The post Syncromune and Eucure enter antibody licence deal appeared first on Pharmaceutical Technology.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

JAMA Internal Medicine
NOVEMBER 23, 2020
Widespread availability of commercial assays that detect anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies has enabled researchers to examine naturally acquired immunity to coronavirus disease 2019 (COVID-19) at the population level.
Pharmaceutical Technology
AUGUST 15, 2022
The updated, Omicron-containing bivalent vaccine that acts on two coronavirus variants is indicated as a booster dose for active immunisation for the prevention of Covid-19 in people of this age group. In baseline seronegative subjects, the updated vaccine offered a superior neutralising antibody response against Omicron (BA.1)

XTalks
NOVEMBER 4, 2020
It has long been known that mothers greatly influence the development of the growing fetus by not only providing nutrients through the placenta, but also a growing list of biological elements including beneficial antibodies, gut bacteria and now, allergies. They reported their findings last week in the journal Science.

Pharmaceutical Technology
AUGUST 5, 2022
This list includes the monoclonal antibody (mAb) PRN100 developed at University College London (UCL) and ALX-002, a treatment developed by Allyx Therapeutics that is also being studied for Alzheimer’s disease. Since research needs to be done under containment conditions, samples cannot be moved across different facilities, he explains.

Scienmag
AUGUST 31, 2020
NEW YORK, NY–A new study of the antibodies produced by people with gluten sensitivity may lead to a better way to detect the condition and treat it.

Pharmaceutical Technology
MARCH 10, 2023
Pharmaceutical and biotechnology industries widely use freeze-drying systems to protect vaccines, antibodies, antibiotics such as penicillin, blood plasma, proteins, enzymes, hormones, viruses, and bacteria from heat and minimise their biological activity. The process eliminates the disadvantages of conventional drying methods or freezing.

Pharmaceutical Technology
JUNE 24, 2022
1 variant, on 22 June, Moderna said its booster also showed a “potent” antibody response against Omicron subvariants BA.4 The mRNA-1273.214 booster contains the original Spikevax vaccine and a candidate targeting Omicron BA.1 In addition to providing protection against the Omicron BA.1 1 variant of concern.

Pharmaceutical Technology
APRIL 4, 2023
The combination therapy can be used to treat la/mUC patients who do not qualify for cisplatin-containing chemotherapy. Merck stated that the regulatory approval represents the first of its kind received by an anti-PD-1 therapy in combination with an antibody-drug conjugate for use in targeted patients in the US.

The Pharma Data
APRIL 18, 2022
The antibodies generated by Pfizer’s COVID-19 vaccine rise more slowly and decline more quickly than those generated by the Moderna vaccine, according to a new study from UVA Health. The researchers determined that both vaccines generated similar peak levels of COVID-fighting antibodies. ” Tracking the COVID-19 Vaccines.
Pharmaceutical Technology
FEBRUARY 15, 2023
Adeno-associated virus vectors, alcohol dehydrogenase compositions, and antibody serum stabilisers are some of the accelerating innovation areas, where adoption has been steadily increasing. Among maturing innovation areas are anti-influenza antibody compositions and anti-interleukin-1, which are now well established in the industry.

The Pharma Data
SEPTEMBER 11, 2021
Boehringer Ingelheim believes Twist’s ability to generate potent, diverse therapeutic antibodies by mining its comprehensive libraries, combined with our extensive capabilities and experience in drug discovery and development, will enable us to deliver breakthrough opportunities to patients,” said Clive R. About Twist Bioscience Corporation.

XTalks
FEBRUARY 23, 2022
The modular production units are housed in containers that come equipped with raw starting materials and state-of-the-art technology for producing mRNA-based vaccines from start to finish, except for the fill-and-finish step, which will be carried out by local manufacturers.

Pharmaceutical Technology
FEBRUARY 25, 2023
The therapeutic candidate is a bispecific antibody targeting CD3 and claudin 18.2 Xencor overview Xencor is a clinical stage biopharmaceutical company that focused on discovering and developing engineered monoclonal antibody and cytokine therapeutics to treat patients with cancer and autoimmune diseases.

Pharmaceutical Technology
FEBRUARY 25, 2023
The therapeutic candidate is a bispecific antibody targeting CD3 and claudin 18.2 Xencor overview Xencor is a clinical stage biopharmaceutical company that focused on discovering and developing engineered monoclonal antibody and cytokine therapeutics to treat patients with cancer and autoimmune diseases.

Pharmaceutical Technology
FEBRUARY 25, 2023
The therapeutic candidate is a bispecific antibody targeting CD3 and claudin 18.2 Xencor overview Xencor is a clinical stage biopharmaceutical company that focused on discovering and developing engineered monoclonal antibody and cytokine therapeutics to treat patients with cancer and autoimmune diseases.

Scienmag
MARCH 30, 2022
The New England Journal of Medicine (NEJM) today published final results of a nationwide multicenter study led by researchers at Johns Hopkins Medicine and the Johns Hopkins Bloomberg School of Public Health that show plasma from patients who have recovered from COVID-19 and whose blood contains antibodies against SARS-CoV-2, the causative virus, is (..)

BioSpace
MAY 5, 2021
FDA approved Keytruda combined with Genentech’s Herceptin (trastuzumab), fluoropyrimidine- and platinum-containing chemotherapy, for first-line treatment of locally advanced unresectable or metastatic HER2-positive gastric or gastroesophageal junction adenocarcinoma.

Pharmaceutical Technology
JUNE 5, 2023
ITM-11 is a targeted radionuclide therapy, where patients are injected with small amounts of radiopharmaceuticals that contain a targeting molecule, like antibodies or peptides, and a medical radioisotope. ITM-11 consists of the octreotide-derived somatostatin analog edotreotide and EndolucinBeta.

The Pharma Data
NOVEMBER 25, 2020
26, 2020 /PRNewswire/ — Cantargia AB (OMXS: CANTA) and BioInvent International AB (OMXS: BINV), today announced that BioInvent has been contracted as manufacturer of Cantargia’s antibody CAN10 in preclinical development for the treatment of systemic sclerosis and myocarditis. LUND, Sweden , Nov. About BioInvent. About Cantargia.
XTalks
JANUARY 21, 2025
The liquid portion of the blood (serum or plasma) containing antibodies is separated and tested using the Liason Biotrin parvovirus B19 IgG plus test on a Liason XL analyzer. If parvovirus B19 antibodies are present, they bind to the chemicals and particular particles in the test, producing a light signal.

The Pharma Data
SEPTEMBER 17, 2020
The new Elecsys Anti-SARS-CoV-2 S test can quantitatively measure the level of antibodies against SARS-CoV-2 in patients who have been exposed to the virus. The test targets antibodies against the spike protein. The majority of current candidate vaccines aim to induce an antibody response against the spike protein. “As

Medical Xpress
DECEMBER 1, 2022
In 2021, a group of scientists led by researchers at the University of North Carolina at Chapel Hill, Weill Cornell Medicine and NewYork-Presbyterian reported that the Moderna mRNA vaccine and a protein-based vaccine candidate containing an adjuvant, a substance that enhances immune responses, elicited durable neutralizing antibody responses to SARS-CoV-2 (..)
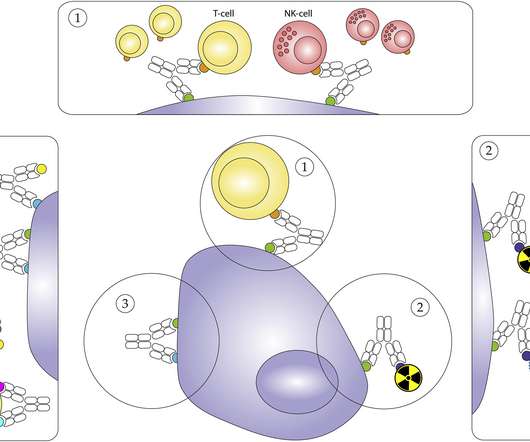
pharmaphorum
NOVEMBER 2, 2021
Dr Laura Moriarty, senior marketing manager at Bio-Rad, looks at the impressive immuno-therapeutic potential of bispecific antibodies (bsAbs). Though their bispecific nature complicates large-scale production and purification workflows, with challenges such as antibody chain mispairing, bsAbs have come a long way since first developed.
The Pharma Data
DECEMBER 11, 2020
The test has been designed to quantitatively measure of immunoglobulin G (IgG) antibodies to aid clinicians in identifying patients with an immune Covid-19 response pre- and post-vaccination. As we follow individuals over the course of a year, we are repeatedly testing them for both virus and antibodies.”.

Worldwide Clinical Trials
SEPTEMBER 29, 2022
For studies of humanized therapeutics (monoclonal antibodies [mAb], fusion protein, antibody-drug conjugates [ADC], bispecific antibodies, etc.), As such, they employ high dosages, so most samples contain highly concentrated drug levels. anti-human IgG).

The Pharma Data
SEPTEMBER 17, 2020
17, 2020 /PRNewswire/ — Eli Lilly and Company (NYSE:LLY) and Amgen (NASDAQ:AMGN) today announced a global antibody manufacturing collaboration to significantly increase the supply capacity available for Lilly ‘s potential COVID-19 therapies. INDIANAPOLIS and THOUSAND OAKS, Calif. Reese , M.D.,

The Pharma Data
JANUARY 11, 2021
a San Diego-based biotechnology company with an array of technology platforms for antibody discovery and optimization, and novel NK and T cell engager generation, today announced licensing of a panel of its anti-SARS-CoV-2 antibody clones to IGM Biosciences for COVID-19 therapy development.

Scienmag
OCTOBER 16, 2020
The blood of recovered patients contains antibodies that act against the coronavirus. Washington, DC – October 16, 2020 – In the absence of approved, effective treatments for COVID-19, some hospitals have been treating patients with severe COVID symptoms with blood plasma from recovering patients.

BioSpace
JUNE 7, 2022
Moderna announced its Omicron-containing COVID-19 booster candidate, mRNA-1273.214, demonstrated superior antibody response against Omicron in its Phase II/III study.

Pharmaceutical Technology
SEPTEMBER 15, 2022
The increase in the production of biological drugs such as vaccines, gene and cell therapies, and monoclonal antibodies, is increasing the volume of injectable drug products in the global healthcare market. Factors driving the demand for injectables.

Pharmaceutical Technology
SEPTEMBER 8, 2022
The information contained within the download document is designed for pharmaceutical executives, developers, research scientists and associates, regulatory solutions consultants, and any other individual involved in API biologics production in the pharmaceutical industry.
Pharmaceutical Technology
FEBRUARY 15, 2023
Adeno-associated virus vectors, alcohol dehydrogenase compositions, and antibody serum stabilisers are some of the accelerating innovation areas, where adoption has been steadily increasing. Among maturing innovation areas are anti-influenza antibody compositions and anti-interleukin-1, which are now well established in the industry.

Roots Analysis
DECEMBER 11, 2022
Antibody drug conjugates (ADCs) are an upcoming class of targeted therapeutic agents that have garnered the attention of pharmaceutical companies, and academic / research institutions across the world. Antibody drug conjugates market is presently an established therapeutic concept with 11 approved products.
The Pharma Data
DECEMBER 31, 2020
Antibodies Respond Differently to Severe Versus Mild COVID-19. Researchers at Stanford Medicine found that COVID-19 antibodies preferentially target different parts of the SARS-CoV-2 virus in mild COVID-19 cases than they do in severe cases. People with severe COVID-19 have low proportions of antibodies that target the spike protein.

Camargo
NOVEMBER 29, 2021
Essentially, a biologic is a product that is produced from living organisms or that contains components of living organisms. Monoclonal antibodies (mAbs). applicable to the prevention, treatment, or cure of a disease or condition of human beings.”. Biologics include a wide range of products , including: Vaccines. Growth factors.

The Pharma Data
JANUARY 12, 2021
million additional doses of the casirivimab and imdevimab antibody cocktail, bringing total potential U.S. Under the new agreement, the government will purchase all finished doses of the casirivimab and imdevimab antibody cocktail delivered by June 30, 2021 , up to 1.25 TARRYTOWN, N.Y. , 12, 2021 /PRNewswire/ — . million doses.

XTalks
JANUARY 31, 2022
The third group will contain unimmunized individuals who will get three doses of the omicron shot. Neutralizing Antibody Data for Omicron Targeting. The study showed that antibody neutralization against omicron decreased by 6.3-fold Moderna will be evaluating its omicron targeting booster through extension of an earlier study.
pharmaphorum
JULY 27, 2022
Clinical data show strong neutralising antibody responses against Omicron BA.1, 1-containing boosters, but last month the FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) concluded that future booster campaigns against COVID-19 should ideally include vaccines with a component targeting BA.4

Camargo
DECEMBER 13, 2021
An application submitted under 351(a), also known as a “stand-alone” application, must contain all safety and effectiveness information for a biological product and cannot depend on any other biological product. One common characteristic for most if not all biologics is the triggering of an immune response or anti-drug antibodies.

The Pharma Data
DECEMBER 29, 2020
First antibody therapy to demonstrate anti-viral effect in patients hospitalized with COVID-19. NASDAQ: REGN) today announced encouraging initial data from an ongoing Phase 1/2/3 clinical trial of the Regeneron antibody cocktail, casirivimab and imdevimab, in hospitalized COVID-19 patients requiring low-flow oxygen. futility analysis).

The Pharma Data
JANUARY 12, 2021
LONDON, UK / ACCESSWIRE / January 13, 2021 / Hemogenyx Pharmaceuticals plc (LSE:HEMO), the biopharmaceutical group developing new therapies and treatments for blood diseases, is pleased to announce that it has successfully completed the development of its CDX antibody with a leading global pharmaceutical company (“GlobalCo”).

The Pharma Data
NOVEMBER 20, 2020
NASDAQ: REGN) today announced that the antibody cocktail casirivimab and imdevimab administered together (formerly known as REGN-COV2 or REGEN-COV2), a therapy currently being investigated for use in COVID-19 , has received Emergency Use Authorization (EUA) from the U.S. TARRYTOWN, N.Y., November 21, 2020 – Regeneron Pharmaceuticals, Inc.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content