Humanised bispecific antibody for asthma enters Phase I trial
Drug Discovery World
MARCH 8, 2024
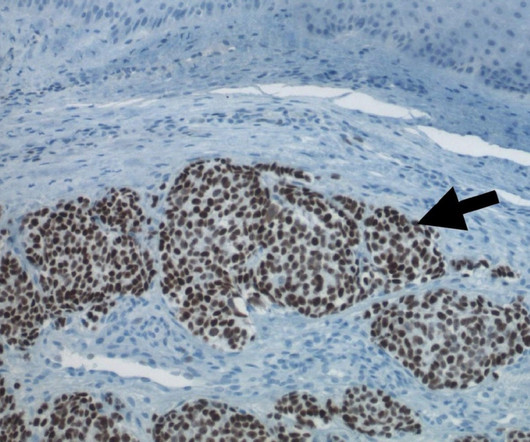
Biopharmaceutical company Innovent Biologics has launched the first-in-human (FIH) Phase I clinical trial of IBI3002, a novel bispecific antibody targeting Interleukin 4 receptor α (IL-4Rα) and thymic stromal lymphopoietin (TSLP). In vitro assays have shown superiority over the marketed monoclonal antibodies to respective target.









































Let's personalize your content