Supply shortage hits infant RSV antibody Beyfortus
Bio Pharma Dive
OCTOBER 25, 2023
The CDC is asking doctors to ration supply of Sanofi and AstraZeneca’s new RSV drug Beyfortus, as demand has outstripped supply.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
pharmaphorum
AUGUST 21, 2020
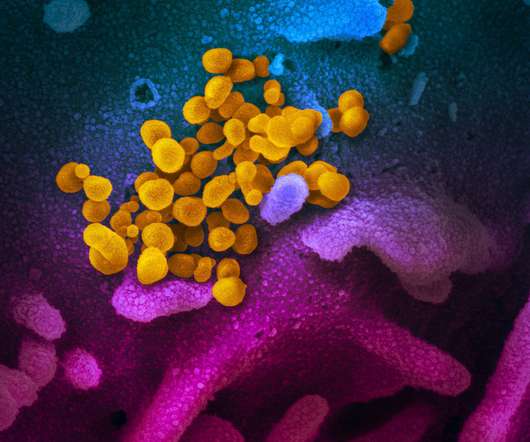
Doctors have called for rules to be tightened on coronavirus antibody tests in the UK amid fears that false readings could put the public at risk. It’s not even known for sure that having antibodies against the SARS-CoV-2 coronavirus confers immunity. Feature image courtesy of Rocky Mountain Laboratories/NIH.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

pharmaphorum
FEBRUARY 10, 2021
The FDA has granted an emergency licence for Eli Lilly’s COVID-19 antibody combination therapy, to reduce chances of high-risk patients progressing from mild to moderate disease to more severe symptoms that may need hospital treatment. There are a range of other antibody therapies in various stages of development.

pharmaphorum
SEPTEMBER 16, 2020
Regeneron’s COVID-19 antibody cocktail has been added to the UK’s RECOVERY trial, one of the largest of its kind in the world that is testing several potential coronavirus therapies at the same time. REGN-COV2 is a combination of two monoclonal antibodies, designed to block infections of SARS-CoV-2, the virus that causes COVID-19.

STAT News
JULY 22, 2022
So Padgett was shocked last month when, during a Google search on new variants, she learned there was a drug available to protect patients like her daughter, a 23-year-old childhood cancer survivor whose immune system can’t make enough antibodies. Even more startling: It had been available since December.

Pharmaceutical Technology
FEBRUARY 3, 2023
Being jointly developed by AstraZeneca and Amgen, Tezspire is a first-in-class human monoclonal antibody and inhibits TSLP, an important epithelial cytokine that plays a vital role in multiple inflammatory cascades. Pharyngitis, arthralgia, and back pain are the most common adverse reactions of the antibody.

Scienmag
AUGUST 31, 2020
NEW YORK, NY–A new study of the antibodies produced by people with gluten sensitivity may lead to a better way to detect the condition and treat it.
XTalks
JANUARY 21, 2025
The liquid portion of the blood (serum or plasma) containing antibodies is separated and tested using the Liason Biotrin parvovirus B19 IgG plus test on a Liason XL analyzer. If parvovirus B19 antibodies are present, they bind to the chemicals and particular particles in the test, producing a light signal.

Fierce Pharma
JUNE 1, 2024
Armed with two positive phase 3 trial readouts and the hope that at least one will show a significant patient survival benefit, GSK thinks it has the data to convince doctors and the FDA that the o | Armed with two positive phase 3 trial readouts and the hope that at least one will show a significant patient survival benefit, GSK thinks it has the (..)

The Pharma Data
APRIL 18, 2022
The antibodies generated by Pfizer’s COVID-19 vaccine rise more slowly and decline more quickly than those generated by the Moderna vaccine, according to a new study from UVA Health. The researchers determined that both vaccines generated similar peak levels of COVID-fighting antibodies. ” Tracking the COVID-19 Vaccines.

The Pharma Data
AUGUST 20, 2020
Poor regulation of antibodies tests – that could indicate if someone has had coronavirus – could be putting the public at risk, doctors have warned. It is still not known whether having antibodies will protect people from a second infection. Coronavirus infection triggers the immune system to produce antibodies.

BioTech 365
NOVEMBER 4, 2021
City of Hope Doctors Present New Research on Cancer Immunotherapies City of Hope Doctors Present New Research on Cancer Immunotherapies New data on bispecific antibodies and stem cell transplants will be presented in person and virtually at the American Society … Continue reading →

Pharmaceutical Technology
FEBRUARY 1, 2023
Since the FDA approved AstraZeneca’s monoclonal antibody therapy Synagis (palivizumab) 25 years ago, drug development successes for RSV have been few and far between. The two types of candidates currently in the pipeline can be categorised as monoclonal antibodies and vaccines.
World of DTC Marketing
SEPTEMBER 14, 2021
Even if humoral immunity appears to wane, reductions in neutralizing antibody titre do not necessarily predict reductions in vaccine efficacy over time, and reductions in vaccine efficacy against mild disease do not necessarily predict reductions in the (typically higher) efficacy against severe disease.

World of DTC Marketing
MARCH 16, 2021
Do I have a responsibility to share — on every occasion, random and formal, to family, friends, and strangers alike — my knowledge on the vaccines, monoclonal antibodies, the latest news from the Lancet or JAMA, why RNA is not voodoo, how Phase 4 safety trials are happening as I write…The answer is simple: yes.

Scienmag
JANUARY 22, 2021
Nearly pain-free microneedle patch can test for antibodies and more in the fluid between cells Credit: Image: Sisi Cao Blood draws are no fun. Oftentimes, doctors use blood samples to check for biomarkers of disease: antibodies that signal […].

The Pharma Data
APRIL 22, 2023
Cocktail of modified antibodies provides strong effect against SARS-CoV-2 Is it possible to improve the antibodies that the body produces to fight SARS-CoV2? In a study led by researchers from Lund University in Sweden, this was investigated by redesigning antibodies and combining them against the virus.

pharmaphorum
FEBRUARY 26, 2021
A former NHS doctor, he noted how stressful and draining some days can be when working at healthcare’s frontline – even in normal times, and that the current fight against the pandemic brings little respite to staff.

Rethinking Clinical Trials
JANUARY 17, 2024
The first monoclonal antibody (mABs) treatment for treatment of COVID-19 was approved in November 2020. UPMC built upon the success of the Remdesivir lottery and in December 2020, UPMC began infusing monoclonal antibodies across 45 UPMC locations. Only about 40% of our patients are enrolled in My UPMC.

pharmaphorum
NOVEMBER 4, 2020
Novartis’ injectable migraine prevention antibody Aimovig has been shown to be more effective than topiramate – a go-to oral therapy for people with chronic migraine – in a head-to-head trial.

pharmaphorum
JUNE 13, 2022
ADC Therapeutics is looking to add a second approved antibody-drug conjugate to its portfolio after reporting strong phase 2 results with camidanlumab tesirine (cami) in relapsed/refractory Hodgkin lymphoma at the EHA annual meeting. “These results offer hope to patients and doctors who need a new option.”

The Pharma Data
NOVEMBER 9, 2020
Food and Drug Administration (FDA) today granted Emergency Use Authorization (EUA) for Eli Lilly and Company’s (NYSE: LLY) investigational neutralizing antibody bamlanivimab (LY-CoV555) 700 mg. INDIANAPOLIS, Nov. 9, 2020 /PRNewswire/ — The U.S. Ricks, Lilly’s chairman and CEO.

XTalks
JULY 8, 2021
The drugs are monoclonal antibodies against the IL-6 receptor. Monoclonal antibodies generally do not come cheap. Despite the recent authorizations and recommendations, many doctors had already been using these drugs as a last resort for severely ill COVID-19 patients, according to Fierce Pharma. We must urgently change this.”.

pharmaphorum
OCTOBER 7, 2020
A BBC report says that at least one NHS trust has advised doctors to ration non-urgent tests and prioritise supply of swabs for coronavirus testing, and there are concerns some essential items could be out of supply within a few days. Antibody test order.

The Pharma Data
JANUARY 26, 2021
. “These exciting results, which replicate positive Phase 2 data in a much larger set of patients, add valuable clinical evidence about the role neutralizing antibodies can play in fighting this pandemic. Lilly’s chief scientific officer and president of Lilly Research Laboratories. have reached record highs.
pharmaphorum
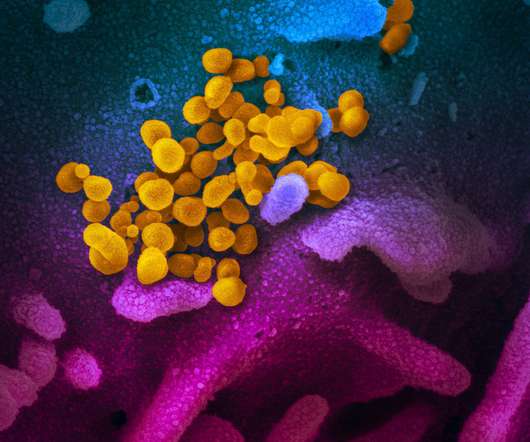
SEPTEMBER 7, 2020
Preliminary results published in The Lancet are from a non-randomised phase 1/2 trial of 76 people aged 18-60, which found an antibody response within 21 days and no serious adverse events after 42 days. All participants in the phase 2 trials (40 participants) produced antibodies against the SARS-CoV-2 spike protein.

pharmaphorum
JANUARY 15, 2023
Yet, according to NMO UK , others might also have antibodies recognised as being associated with this disorder, such as Aquaporin 4 (AQP4) and Myelin Oligodendrocyte Glycoprotein (MOG). However, each person with NMOSD will experience different symptoms, and each will require individually tailored care and support. Patterson explained.

The Pharma Data
JANUARY 15, 2021
Molecular and antigen tests check for active infections, while antibody tests show whether a person has been infected in the past. Your child’s doctor can explain what each test does and when tests can be most useful. There are three main types of COVID-19 tests. © 2021 HealthDay.

pharmaphorum
DECEMBER 21, 2020
The body produces antibodies against the protein, which neutralise the virus in the event of an infection. Moderna’s founders were led by Derrick Rossi, a post doctorate fellow from Stanford University who championed the technology to a team from Flagship Ventures, a Massachusetts firm founded and run by Noubar Afeyan.
pharmaphorum
APRIL 20, 2021
The cost-effectiveness agency has said that anti-CD20 antibody Kesimpta (ofatumumab) can be prescribed via the NHS in England and Wales as a treatment for adults with RMS with active disease, as either a first-line therapy or after alternative drugs have been tried.
XTalks
JUNE 30, 2023
Severe hemophilia A is usually treated with FVIII replacement therapy or an antibody-based drug to improve the ability of blood to clot and reduce the likelihood of bleeding. To be eligible for the therapy, patients must test negative for AAV5-targeted antibodies.

Delveinsight
OCTOBER 1, 2021
This disease is associated with antibodies directed against the acetylcholine receptor, muscle-specific kinase (MUSK), lipoprotein-related protein 4 (LRP4), or agrin in the postsynaptic membrane at the neuromuscular junction. This medication is administered by injection under the skin or slowly into a vein as directed by the doctor.

pharmaphorum
SEPTEMBER 13, 2021
The partnership centres on RGX314, which delivers a gene coding for an antibody against VEGF – a well-established approach to treating wet AMD and the target of current blockbuster drugs like Bayer’s Eylea (aflibercept) and Novartis/Roche’s Lucentis (ranibizumab). AbbVie – which is also putting up $1.38

pharmaphorum
SEPTEMBER 16, 2020
Patients with SEA can be put on a biologic drug to reduce inflammation in the airways, and there are a number of monoclonal antibody drugs available or in development. In SEA, there are higher numbers of eosinophils in the blood, lung tissue and mucus, meaning the whole respiratory tract suffers from airflow obstruction. Impact on daily life.

The Pharma Data
AUGUST 16, 2020
Dr James Hindley says T cells can create more antibodies if a person is exposed to the virus again. A company in Cardiff has developed a test for coronavirus T cells – which can potentially provide longer-term immunity to the virus than antibodies. It has also tested people who have never had coronavirus, to ensure its accuracy.

The Pharma Data
AUGUST 11, 2020
Exposure to Covid-19 is similar in Stockholm and London, based on antibody tests, despite different lockdown strategies, research suggests. Health experts predicted that 40% of the population in the capital, Stockholm, would have developed antibodies to the disease by May. The actual figure was 17%, according to a review of evidence.

The Pharma Data
NOVEMBER 4, 2020
If your doctor decided that urgent treatment with ULTOMIRIS is needed, you should receive meningococcal vaccination as soon as possible. Your doctor will decide if you need additional vaccination. Your doctor will give you a Patient Safety Card about the risk of meningococcal infection.

pharmaphorum
JUNE 24, 2021
Roche bought ex-US rights to the PDS version from Novartis in 2019, giving it total control of the implant product, while Novartis has focused on the development of longer-acting VEGF antibody Beovu (brolucizumab), which is dosed every two or three months. The post Roche eyes October decision by FDA for wet AMD implant appeared first on.

Advarra
MARCH 10, 2023
A visit to a podiatrist and family doctor yielded less than satisfactory diagnoses. One surgery later, Moss spoke with a second doctor who immediately identified it as a sickle cell ulcer, and it would’ve gone away on its own. Moss has had many instances where she had to educate her health care providers on her disease.

Pharmaceutical Technology
OCTOBER 5, 2022
It is also important to ask whether doctors are being paid fair market value and if they are providing the services for which they’re being compensated, he adds. BIIB107 is a monoclonal antibody that targets ?4 At the same time, companies need to prioritize their compliance programs. Biogen’s activity in multiple sclerosis.

pharmaphorum
SEPTEMBER 8, 2021
. “Because NHS staff and resources are scarce, another national mobilisation would potentially leave us with fewer resources for cancer screenings and the other care provided by doctors and nurses each day,” says the AZ executives. ” The post AZ CEO Soriot urges caution on COVID booster doses appeared first on.

XTalks
OCTOBER 26, 2020
” “We are proud to launch these complementary campaigns and be part of the T1D ecosystem empowering doctors and patients with information,” said Eleanor (Leni) Ramos, MD, CMO, Provention Bio. “We She said that’s why it’s “so important that we educate the market with campaigns like the ones we’ve launched.”
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?




Let's personalize your content