FDA approves Regeneron antibody drug as first Ebola virus treatment
Bio Pharma Dive
OCTOBER 14, 2020
Research for the drug, now cleared as Inmazeb, helped Regeneron jumpstart its fast development of an antibody therapy for COVID-19.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Bio Pharma Dive
OCTOBER 14, 2020
Research for the drug, now cleared as Inmazeb, helped Regeneron jumpstart its fast development of an antibody therapy for COVID-19.

Pharmaceutical Technology
JANUARY 9, 2023
Eisai and Biogen have received approval for their antibody Leqembi (lecanemab-irmb) , 100mg/mL injection for intravenous use, from the US Food and Drug Administration (FDA) under the Accelerated Approval Pathway to treat Alzheimer’s disease (AD).
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
SEPTEMBER 11, 2023
The HER3-targeting treatment could become the next antibody-drug conjugate to emerge from Daiichi Sankyo’s laboratories, after the AstraZeneca-partnered Enhertu.
Bio Pharma Dive
SEPTEMBER 21, 2021
Tivdak, a type of drug known as an antibody-drug conjugate, is the fourth approved medicine for Seagen and its third to reach market since December 2019.

Bio Pharma Dive
SEPTEMBER 29, 2020
The world's attention will be on the FDA as it considers initial data from coronavirus vaccine developers. But several other important drugs, including a CAR-T therapy and an Ebola antibody, will also be on the agency's agenda.

Pharmaceutical Technology
AUGUST 12, 2022
The US Food and Drug Administration (FDA) has granted approval for Daiichi Sankyo and AstraZeneca ’s Enhertu (fam-trastuzumab deruxtecan-nxki) to treat unresectable or metastatic non-small cell lung cancer (NSCLC) in adults. In the trial, 1.9%

Bio Pharma Dive
DECEMBER 17, 2024
An FDA approval of Merck’s drug clesrovimab by June would give physicians another option for protecting newborns from respiratory syncytial virus.
Bio Pharma Dive
AUGUST 14, 2023
Elrexfio is the third bispecific antibody cleared to treat the blood cancer, and will compete with other therapies that target the “BCMA” protein on myeloma cells.

Bio Pharma Dive
JULY 17, 2023
Developers AstraZeneca and Sanofi expect to make the preventive antibody treatment, called Beyfortus, available ahead of the upcoming cold season.

Pharmaceutical Technology
AUGUST 8, 2022
AstraZeneca and Daiichi Sankyo ’s Enhertu (trastuzumab deruxtecan) has received expanded approval from the US Food and Drug Administration (FDA) to treat adults with unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer.
Pharmaceutical Technology
NOVEMBER 18, 2022
The US Food and Drug Administration (FDA) has approved Provention Bio’s biologics licence application (BLA) for Tzield (teplizumab-mzwv) to treat type 1 diabetes (T1D) patients. The post US FDA approves Provention Bio’s Tzield to delay diabetes appeared first on Pharmaceutical Technology.

Bio Pharma Dive
JUNE 16, 2023
The approval of Columvi adds another “bispecific antibody” to the market, highlighting the fast research progress for drugs that target two proteins rather than one.

Bio Pharma Dive
MAY 17, 2024
Imdelltra, a bispecific antibody targeting a protein called DLL3, is cleared for use following chemotherapy in treating extensive-stage small cell lung cancer.

Pharmaceutical Technology
MARCH 23, 2023
The US Food and Drug Administration (FDA) has approved Incyte ’s Zynyz (retifanlimab-dlwr) to treat metastatic or recurrent locally advanced merkel cell carcinoma (MCC) in adult patients. Zynyz (retifanlimab-dlwr) is a humanised monoclonal antibody targeting programmed death receptor-1 (PD-1).
Pharmaceutical Technology
FEBRUARY 19, 2024
An FDA approval in late 2024 would make it the first TROP2-directed therapy for NSCLC antibody drug conjugate patients.

Pharmaceutical Technology
FEBRUARY 3, 2023
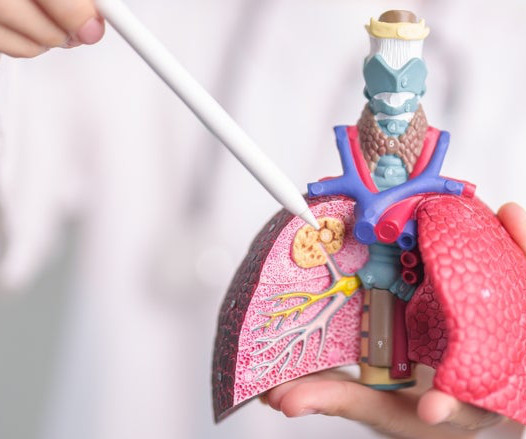
The US Food and Drug Administration (FDA) has granted approval to Amgen and AstraZeneca ’s Tezspire (tezepelumab-ekko) for self-administration using a single-use, pre-filled pen for severe asthma patients aged 12 years and above. Pharyngitis, arthralgia, and back pain are the most common adverse reactions of the antibody.
Pharmaceutical Technology
SEPTEMBER 27, 2024
The US Food and Drug Administration (FDA) has announced the approval of Sanofi and Regeneron’s interleukin (IL)-4 and IL-13 monoclonal antibody, Dupixent, for the US market.

pharmaphorum
DECEMBER 19, 2023
Big pharma companies like Pfizer are making significant investments in antibody-drug conjugates, a promising class of therapies. This article explores the growing interest and recent developments, including FDA approvals and the potential impact on the pharmaceutical industry.

Pharmaceutical Technology
OCTOBER 10, 2022
The US Food and Drug Administration (FDA) has approved GlaxoSmithKline ’s (GSK) Boostrix (tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine adsorbed [Tdap]) for pregnant women during their third trimester to prevent pertussis (whooping cough) in newborn infants.
Pharmaceutical Technology
OCTOBER 7, 2022
Provention Bio and Sanofi US have signed a co-promotion agreement to launch the former’s lead investigational drug candidate, teplizumab for the delay in the onset of clinical type 1 diabetes (T1D). The US Food and Drug Administration (FDA) is presently reviewing teplizumab for the delay of clinical T1D in people who are at risk.

pharmaphorum
DECEMBER 30, 2021
Johnson & Johnson’s much-touted crop of bispecific antibodies for cancer generated its first commercial product in May, and the drugmaker has now filed for FDA approval of a second candidate. The post J&J goes after another FDA approval for a cancer bispecific appeared first on.

Pharmaceutical Technology
MAY 22, 2023
The US Food and Drug Administration (FDA) has granted approval to AbbVie and Genmab ‘s Epkinly (epcoritamab-bysp) to treat relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) in adult patients. The FDA approval of Epkinly represents a new treatment mechanism of action for third-line DLBCL patients. “As
Pharmaceutical Technology
MAY 25, 2023
Celltrion USA has received approval from the US Food and Drug Administration (FDA) for Humira (adalimumab) biosimilar, Yuflyma (adalimumab-aaty) , for multiple indications. Yuflyma is a recombinant fully human anti–tumour necrosis factor α (anti-TNFα) monoclonal antibody.

XTalks
OCTOBER 22, 2024
Astellas Pharma has won approval from the US Food and Drug Administration (FDA) for Vyloy (zolbetuximab) as a first-line treatment for adults with advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma. The drug is specifically indicated for patients whose tumors are HER2-negative and express claudin 18.2
Pharmaceutical Technology
NOVEMBER 14, 2022
The US Food and Drug Administration (FDA) has granted accelerated approval for ImmunoGen’s Elahere (mirvetuximab soravtansine-gynx) to treat adults with folate receptor alpha (FR?)-positive, binding antibody, a cell-surface protein which is greatly expressed in ovarian cancer, as well as the maytansinoid payload DM4.

BioSpace
APRIL 8, 2024
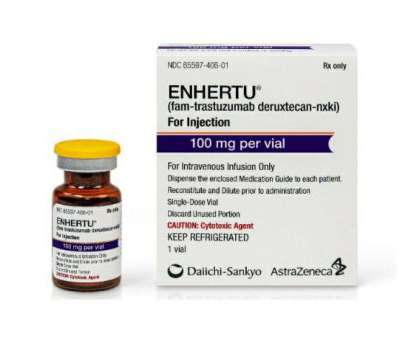
AstraZeneca and Daiichi Sankyo’s antibody-drug conjugate Enhertu is the first FDA-approved tumor-agnostic HER2-targeted therapy authorized for the treatment of solid tumors in adults who have undergone prior systemic treatment.

Pharmaceutical Technology
APRIL 6, 2023
The difficulty in finding a treatment for Alzheimer’s disease has plagued the drug industry for decades. A recent wave of monoclonal antibodies, including the FDA-approved Eisai / Biogen ’s Leqembi (lecanemab), is expected to shake up the space.
pharmaphorum
OCTOBER 4, 2022
The approval brings hope to many, with breast cancer having surpassed lung cancer to become the most commonly diagnosed cancer in the world: there are approximately 2.3 The post Roche gets FDA approval for HER2 breast cancer diagnostic appeared first on. HER2 is a receptor protein that accelerates cancer cell growth.

Pharmaceutical Technology
MAY 19, 2023
The US Food and Drug Administration (FDA) has approved AbbVie’s Rinvoq (upadacitinib) for patients with Crohn’s disease who do not respond to TNF blockers, a common immune suppressant treatment for the condition.

Pharmaceutical Technology
MAY 16, 2023
Health Canada has accepted a new drug submission (NDS) for Eisai and Biogen ’s lecanemabto to treat early Alzheimer’s disease (AD). Lecanemab is an investigational anti-amyloid beta (Aβ) protofibril antibody directed against aggregated soluble (protofibril), as well as insoluble forms of Aβ.
Pharmaceutical Technology
JUNE 15, 2023
Alentis Therapeutics has received US Food and Drug Administration (FDA) clearance for its investigational new drug (IND) application for ALE.C04 to treat Claudin-1 positive (CLDN1+) tumours. ALE.C04 is an investigational antibody designed to target exposed CLDN1 in cancer cells.

XTalks
OCTOBER 22, 2021
The US Food and Drug Administration (FDA) has approved Cyltezo (adalimumab-adbm) as the first interchangeable biosimilar for Humira (adalimumab) for the treatment of several inflammatory conditions. This means AbbVie, maker of the world’s top-selling drug Humira, could be in for some stiff competition.

Pharmaceutical Technology
FEBRUARY 5, 2023
The US Food and Drug Administration (FDA) has granted approval for Gilead Sciences ’ Trodelvy (sacituzumab govitecan-hziy) to treat unresectable locally advanced or metastatic hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative (IHC 0, IHC 1+ or IHC 2+/ISH–) breast cancer adult patients.
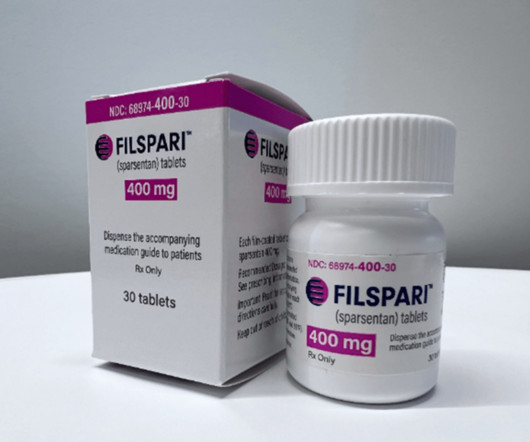
XTalks
MARCH 9, 2023
Travere Therapeutics recently announced that their medication Filspari (sparsentan) received accelerated approval from the US Food and Drug Administration (FDA). The approval was granted for its use as a non-immunosuppressive therapy for the reduction of proteinuria (protein in urine) in IgA nephropathy in adults.

XTalks
DECEMBER 18, 2024
The US Food and Drug Administration (FDA) has granted approval to Checkpoint Therapeutics PD-L1 inhibitor Unloxcyt (cosibelimab-ipdl) for the treatment of metastatic or locally advanced cutaneous squamous cell carcinoma (cSCC) in patients who cannot be cured with surgery or radiation therapy.
XTalks
JULY 25, 2023
Emergent BioSolutions, a multinational specialty biopharmaceutical company headquartered in Gaithersburg, Maryland, has achieved a significant milestone with the approval of its Cyfendus (Anthrax Vaccine Adsorbed, Adjuvanted) vaccine by the US Food and Drug Administration (FDA). Overall, 66.3 on Day 64 in the study.

BioPharma Reporter
OCTOBER 15, 2020
Food and Drug Administration (FDA) has approved Regeneronâs Inmazeb for treatment of Ebola. The antibody cocktail was developed using the same ârapid responseâ technologies as Regeneronâs investigational COVID-19 antibody combination.
XTalks
NOVEMBER 30, 2023
Pfizer spinout SpringWorks Therapeutics’ Ogsiveo (nirogacestat) has received US Food and Drug Administration (FDA) approval for the treatment of desmoid tumors, an ultra-rare subtype of non-cancerous soft tissue sarcomas that can cause severe pain and disfigurement.
XTalks
NOVEMBER 16, 2022
has won US Food and Drug Administration (FDA) approval for its antibody-drug conjugate (ADC) Elahere (mirvetuximab soravtansine-gynx) for the treatment of platinum-resistant ovarian cancer. The drug was approved under the FDA’s accelerated pathway and is indicated for adult patients with folate receptor alpha (FR?)-positive

XTalks
AUGUST 14, 2023
The Janssen Pharmaceutical Companies of Johnson & Johnson announced that its bispecific antibody Talvey (talquetamab-tgvs) received US Food and Drug Administration (FDA) approval as a fifth-line treatment for adult patients with heavily pretreated multiple myeloma. Results were similar at the higher 0.8

BioSpace
AUGUST 16, 2020
Food and Drug Administration approved Genentech’s Enspryng (satralizumab-mwge) as a subcutaneous treatment for adults with anti-aquaporin-4 (AQP4) antibody positive neuromyelitis optica spectrum disorder.

Fierce Pharma
AUGUST 10, 2023
Johnson & Johnson is digging its multiple myeloma moat deeper thanks to FDA approval for another novel drug. The FDA has signed off on the company's Talvey, a first-in-class bispecific antibody targeting GPRC5D. Johnson & Johnson is digging its multiple myeloma moat deeper.

XTalks
FEBRUARY 26, 2024
The US Food and Drug Administration (FDA) has granted expanded approval to Genentech’s (part of Roche) Xolair (omalizumab) to help reduce allergic reactions to various foods after accidental exposure.

XTalks
JUNE 8, 2022
Amgen’s Riabni, a CD20-directed cytolytic antibody and biosimilar to Rituxan, got approval from the FDA on Monday for adults with moderate to severe rheumatoid arthritis. B-cell targeted therapy with the monoclonal antibody Rituxan (rituximab) is used to treat RA. million Americans.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content