Krystexxa Chronic Gout Medication Gets Expanded FDA Approval
XTalks
JULY 14, 2022
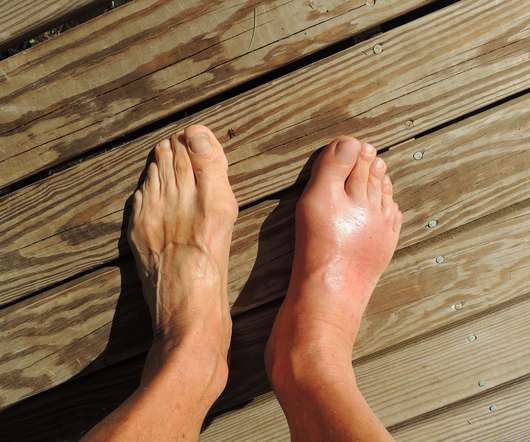
Over time, even the use of Krystexxa can lead to the development of antibodies against it, reducing its effectiveness. Early data also showed that methotrexate could prevent the formation of anti-drug antibodies. Related: Vtama (tapinarof) Cream Gains FDA Approval for the Treatment of Plaque Psoriasis in Adults.



















Let's personalize your content