Twist Bioscience and Bayer partner for antibody discovery
Pharmaceutical Technology
OCTOBER 6, 2023
Genomics company Twist Bioscience has entered into an antibody discovery, option and licence agreement with German drug manufacturer Bayer.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
OCTOBER 6, 2023
Genomics company Twist Bioscience has entered into an antibody discovery, option and licence agreement with German drug manufacturer Bayer.

pharmaphorum
FEBRUARY 18, 2021
GlaxoSmithKline is expanding a coronavirus antibody collaboration with US biotech Vir, to include potential therapies for flu and other respiratory viruses. Several firms are already working on COVID-19 antibody therapies, with Regeneron and Eli Lilly leading the way and AstraZeneca also in the mix with a long-acting antibody.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Medical Xpress
FEBRUARY 17, 2023
A team at UNC Charlotte's Center for Computational Intelligence to Predict Health and Environmental Risks (CIPHER) and Tuple, a Charlotte-based genomics consulting firm, has used artificial intelligence to rapidly assess the public health implications of the newly emergent SARS-CoV-2 XBB.1.5

Pharmaceutical Technology
MAY 12, 2023
The collaboration will use the integrated drug creation platform of Absci and the clinical immunology datasets from the Kennedy Institute to identify antibodies to immune-mediated inflammatory diseases and autoimmune conditions with unprecedented speed.

BioTech 365
DECEMBER 3, 2021
Global Research Antibodies Market Drivers 2021-2028 Include Rise In The Availability Of Technologically Advanced Products and a Rise In Proteomics And Genomics Research – ResearchAndMarkets.com Global Research Antibodies Market Drivers 2021-2028 Include Rise In The Availability Of Technologically Advanced Products … Continue reading → (..)

Drug Discovery Today
FEBRUARY 16, 2021
Celltrion is on track developing a neutralising antibody cocktail with Regdanvimab (CT-P59) tailored to fight against the emerging new variants. CT-P59 demonstrated neutralising capability against key emerging mutations, including the UK variant in addition to six variant genome mutations of SARS-CoV-2.

BioSpace
JUNE 7, 2022
Financing rounds will support platforms for tumor-targeting antibodies, genome-scale nucleic and acid imaging. Funding this week went mostly to companies with sophisticated tech platforms.

pharmaphorum
MARCH 24, 2022
The examples remain few and far between, however, as in recent decades the pharma industry has turned its attention to synthetic molecules and biologics like antibodies. The platform uses an artificial intelligence (AI) algorithm to zero in on the genes encoding natural products based on their computationally predicted human target.

The Pharma Data
SEPTEMBER 11, 2021
Boehringer Ingelheim believes Twist’s ability to generate potent, diverse therapeutic antibodies by mining its comprehensive libraries, combined with our extensive capabilities and experience in drug discovery and development, will enable us to deliver breakthrough opportunities to patients,” said Clive R. About Twist Bioscience Corporation.

pharmaphorum
DECEMBER 5, 2022
The French biotech says VLA1553 met its objective in the phase 3 study, with 99% of patients exhibiting an antibody response against the virus 12 months after receiving a single dose of the shot, with no evidence of a tailing off in levels in the last six months. It will continue for up to five years.
Pharmaceutical Technology
JULY 5, 2022
On leaving the liver cells, the virus hijacks bits of cell membrane and conceals itself from antibodies that would have isolated the virus before it travelled far through the bloodstream. HAV usually hijacks TENT4 and utilises it for the replication of its own genome.

pharmaphorum
OCTOBER 4, 2022
AstraZeneca’s rare disease firm Alexion is set to expand its genomic medicine portfolio with the acquisition of gene editing specialist LogicBio Therapeutics, in a deal worth approximately $68 million. The LogicBio deal is the latest in a string of acquisitions for AstraZeneca.
XTalks
JANUARY 21, 2025
The liquid portion of the blood (serum or plasma) containing antibodies is separated and tested using the Liason Biotrin parvovirus B19 IgG plus test on a Liason XL analyzer. If parvovirus B19 antibodies are present, they bind to the chemicals and particular particles in the test, producing a light signal.

The Pharma Data
OCTOBER 9, 2021
Roche (SIX RO, ROG; OTCQX RHHBY) now posted that gantenerumab, ananti-amyloid beta antibody developed for subcutaneous administration, has been granted Improvement Rectifier Designation by theU.S. Food and Drug Administration (FDA) for the treatment of people living with Alzheimer’s fever ( Notice). Source link: [link].
pharmaphorum
MARCH 28, 2022
Israel-based AION Labs and German independent research institute BioMed X announced its third global call for applications to form a new start-up company focused on using AI to design and optimise antibodies for targeted therapies. . From there, they evaluate what kind of antibodies are produced. ” The Application .

Pharmaceutical Technology
AUGUST 16, 2022
In 2019, Daiichi Sankyo entered a global development and commercialisation agreement with AstraZeneca for Daiichi Sankyo’s lead antibody-drug conjugate (ADC), Enhertu (trastuzumab deruxtecan), in a deal worth $6.9bn. An ORR of 37% was reported for the overall trial population, with this increased to 62% in previously untreated patients.

pharmaphorum
OCTOBER 5, 2022
Sanofi has added to its rare disease pipeline by licensing an antibody-RNA conjugate (ARC) for facioscapulohumeral muscular dystrophy (FSHD), a genetic muscle disorder, from US biotech miRecule.

The Pharma Data
OCTOBER 14, 2021
Roche announced that gantenerumab, an anti-amyloid beta antibody developed for subcutaneous administration, has been granted Breakthrough Therapy Designation by the U.S. In recent years, Roche has invested in genomic profiling and real-world data partnerships and has become an industry-leading partner for medical insights.

pharmaphorum
JANUARY 24, 2023
The cost of testing per human genome in 2006 was approximately $14 million , and in less than two decades, an average consumer-purchased genetic test costs $100. The same is becoming true for the healthcare industry, and one of the first major breakthroughs in the area was the 100,000 Genomes Project.
pharmaphorum
OCTOBER 20, 2021
It will attempt to validate the software by comparing its findings with clinical data such as brain imaging and genomics analyses to see if it can accurately detect neurocognitive impairments that can’t be found using current tests.
pharmaphorum
JULY 14, 2022
The antibody-drug conjugate (ADC) was turned down by NICE in draft guidance published earlier this year, leading to concerns of a disparity in access to the drug as the Scottish Medicines Consortium (SMC) had already cleared its use. NICE estimates that around 2,800 people are eligible for treatment with Piqray plus fulvestrant.

The Pharma Data
JANUARY 24, 2021
Dr. Bond has extensive experience in the discovery and development of adoptive cell therapies, monoclonal antibodies, and cellular engineering and genome editing. As part of his work with Juno and Kite, Dr. Bond led R&D collaborations with genome editing companies including Editas Medicine and Sangamo Therapeutics.

pharmaphorum
JULY 13, 2022
The first subject has now been given a dose of VERVE-101, in what is thought to be the first test in humans of an experimental CRISPR/Cas9 genome editing technique known as base editing. Through base editing, drugs replace single nucleotide in the DNA strand with another, without making double-strand breaks (DSBs) in the gene.

pharmaphorum
DECEMBER 22, 2020
These followed two deals late last week – on Thursday GSK made an $85 million licensing agreement with Surface Oncology for an early-stage antibody asset, which adds a natural killer cell approach to the company’s oncology portfolio. The antibody targets PVRIG, an inhibitory protein expressed on natural killer cells (NK cells) and T-cells.

pharmaphorum
NOVEMBER 12, 2020
In 2018, it bought Element Genomics – a spin-out of Duke University in the US – to add genome-editing technologies, and has also been growing its internal exercise at its Boston R&D hub, although so far it hasn’t advanced and gene therapies into clinical development.

Delveinsight
AUGUST 27, 2020
As observed in 45 younger adults, the middle dose of the vaccine triggered the production of neutralizing antibodies against SARS-CoV-2, the virus, which causes COVID-19 , in 10 patients aged 56 to 70 and 10 patients over 71. Freenome secures USD 270 Million to boost its colorectal cancer blood test.
The Pharma Data
MARCH 20, 2021
The vast majority of current laboratory testing for COVID-19 is based either on detection of viral antigens, nucleic acids or on detection of virus-specific antibodies in the sera of patients. Detection of virus-specific antibodies allows for diagnosis of recent symptomatic or even asymptomatic infections. SARS-CoV-2 proteome.

Pharmaceutical Technology
FEBRUARY 3, 2023
The field of genomic medicine has reached a true turning point. Moving the viral vector field closer to the monoclonal antibody field is really what we see as the way forward.” More efficient, suspension-based cell culture processes are urgently needed in the now fast-developing genomic medicine space.
Pharmaceutical Technology
JUNE 5, 2023
Summit Therapeutics has announced data for its novel investigational bispecific antibody, ivonescimab, known as SMT112 or AK112 in a non-small cell lung cancer study. Among the subset of 63 patients with squamous histology, the progression-free survival was 11 months and 12.3 months for the overall cohort.

The Pharma Data
OCTOBER 14, 2021
In recent years, Roche has invested in genomic profiling and real-world data partnerships and has become an industry-leading partner for medical insights. Roche is also the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a frontrunner in diabetes management. Source link: [link].
Roots Analysis
MARCH 24, 2022
Scientific fields involved in high throughput measurement of biological molecules are referred with the suffix- omics including, transcriptomics, genomics, proteomics and metabolomics. Genomics is the study of genetic material within a cell, and transcriptomics is the study of gene activity in different cells. Web: [link].

pharmaphorum
JULY 27, 2021
He began his career working on the human genome before moving on to screening cell lines for novel antibodies in cancer helping to develop one of the market leading drugs for the disease. The post COVID-19: We don’t need another hero appeared first on.

The Pharma Data
NOVEMBER 5, 2020
Deep Genomics – Toronto-based Deep Genomics appointed Amanda Kay to the newly-created role of chief business officer. Prior to joining Deep Genomics, Kay was senior vice president of Corporate Development and a member of the executive team at Synlogic, Inc. Additionally, the company also appointed Thomas E.

The Pharma Data
NOVEMBER 3, 2020
LogicBio’s proprietary genome editing technology platform, GeneRide, enables the site-specific integration of a therapeutic transgene without nucleases or exogenous promoters by harnessing the native process of homologous recombination.

The Pharma Data
JUNE 7, 2023
Datopotamab deruxtecan is a specifically engineered TROP2 directed DXd antibody drug conjugate (ADC) being jointly developed by Daiichi Sankyo (TSE: 4568) and AstraZeneca (LSE/STO/Nasdaq: AZN). Results will be presented on June 6 during an oral presentation (#9004) at the American Society of Clinical Oncology Annual Meeting (#ASCO23).

The Pharma Data
OCTOBER 14, 2020
Potential Development of Human Monoclonal Antibody Therapeutics to SARS-CoV-2. The research is part of an ongoing collaboration between Columbia University and Tonix that focuses on T cell and antibody responses to SARS-CoV-2 (CoV-2), the virus that causes COVID-19. CHATHAM, N.J., Ilya Trakht, Ph.D.,

XTalks
SEPTEMBER 30, 2020
The research shows that the activity of the immune messenger type 1 interferon (IFN) protein is diminished, either by genetic mutations or an autoimmune attack by neutralizing antibodies against it, in a subset of COVID-19 patients. These rogue antibodies blocked interferon action and were not present in patients with mild COVID-19 cases.
The Pharma Data
MARCH 30, 2021
KEYTRUDA is a humanized monoclonal antibody that blocks the interaction between PD-1 and its ligands, PD-L1 and PD-L2, thereby activating T lymphocytes which may affect both tumor cells and healthy cells. About KEYTRUDA ® (pembrolizumab) Injection, 100 mg. Merck has the industry’s largest immuno-oncology clinical research program.

The Pharma Data
DECEMBER 12, 2020
AstraZeneca has developed a broad range of technologies, initially focused on small molecules and biologics and with a growing focus in precision medicine, genomics, oligonucleotides and epigenetics. Alexion ‘s franchise includes Soliris (eculizumab), a first-in-class anti-complement component 5 (C5) monoclonal antibody.
The Pharma Data
DECEMBER 10, 2020
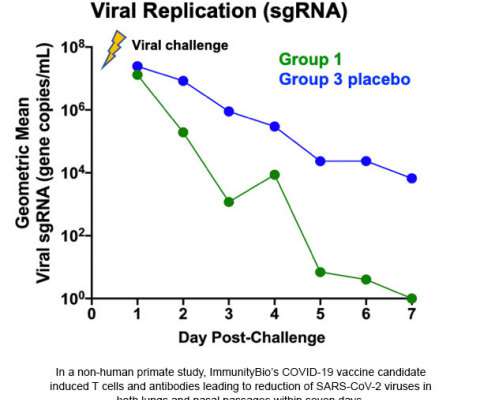
The goal of targeting both S and N was to both activate virus-specific T cells and generate anti-SARS-CoV-2-neutralizing antibodies. ImmunityBio’s hAd5 vaccine generated robust neutralizing antibody activity that was present 14 days post-final vaccination. This press release features multimedia. Graphic: Business Wire).

FDA Law Blog
NOVEMBER 2, 2023
Regarding screening for risk of liver injury: “Total and/or neutralizing antibody titers are screened in many clinical studies, but how such testing is performed, the cut-offs, and the acceptance criteria are all variables that may need standardization.”
The Pharma Data
JULY 6, 2021
They compared these sequences with the original SARS-CoV-2 genome, cross-checking the genetic changes against their highly networked epitopes. The team obtained sequences from the newly circulating B.1.1.7 1.351 Beta, P1 Gamma, and B.1.617.2 Delta SARS-CoV-2 variants of concern.
pharmaphorum
OCTOBER 12, 2022
David Del Bourgo (CEO and co-founder, Whitelab Genomics) has always been passionate about introducing disruptive, innovative technologies to markets. We founded Whitelab Genomics after realising the potential to use data, data science, and AI in a more systematic way to develop genomic therapies,” Del Bourgo says.

The Pharma Data
MARCH 11, 2021
ALIMTA is indicated in combination with pembrolizumab and platinum chemotherapy for the initial treatment of patients with metastatic nonsquamous non-small cell lung cancer, with no EGFR or ALK genomic tumor aberrations. Limitation of Use: ALIMTA is not indicated for the treatment of patients with squamous cell non-small cell lung cancer.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content