EMA CHMP recommends authorisation of AstraZeneca’s Evusheld for Covid-19
Pharmaceutical Technology
SEPTEMBER 19, 2022
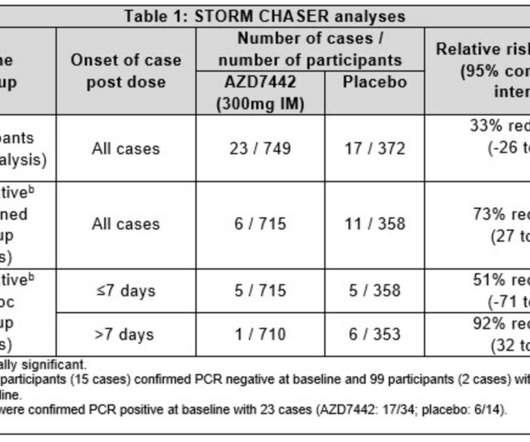
The EMA CHMP positive opinion is based on findings from the double-blind, randomised, placebo-controlled, multicentre Phase III TACKLE clinical trial of IM dose of Evusheld. Additionally, in the trial, the antibody cocktail was found to be well-tolerated. 5 variant, the company noted.






































Let's personalize your content