Twist and Cancer Research Horizons sign antibody library platform deal
Pharmaceutical Technology
JULY 19, 2023
Twist Bioscience and CRUK innovation arm Cancer Research Horizons have signed an agreement for licensing a library of libraries.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
APRIL 21, 2023
Twist Bioscience and Astellas Pharma have entered a collaboration to help the latter to discover antibodies for immunotherapies. In May 2022, they signed a research partnership and exclusive option licence agreement for the development of antibodies to reduce tumour microenvironment-mediated immunosuppression.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
OCTOBER 21, 2022
AbbVie has announced the acquisition of UK-based biotechnology firm DJS Antibodies for nearly $255m in cash at closing. DJS focuses on the discovery and development of antibody therapies that act on difficult-to-drug disease-causing proteins, such as G protein-coupled receptors (GPCRs).

Pharmaceutical Technology
JANUARY 9, 2023
Eisai and Biogen have received approval for their antibody Leqembi (lecanemab-irmb) , 100mg/mL injection for intravenous use, from the US Food and Drug Administration (FDA) under the Accelerated Approval Pathway to treat Alzheimer’s disease (AD). The regulatory approval is based on the data obtained from the Phase II trial.

Pharmaceutical Technology
JULY 1, 2022
Ildong Pharmaceutical will licence a suite of Twist Bioscience's VHH antibody libraries after the two companies entered a partnership agreement. Twist's VHH libraries, used for discovering and developing antibodies for use in immuno-oncology, will be licensed by Ildong for three years for research and development works. .
Pharmaceutical Technology
DECEMBER 26, 2022
LegoChem Biosciences and Amgen have signed a multi-target research collaboration and license agreement to develop antibody-drug conjugates (ADC). Till date, the company has signed a total of 12 ADC licensing deals, worth more than $5bn.

Pharmaceutical Technology
OCTOBER 18, 2022
Gilead Sciences has entered an exclusive option and partnership agreement with MacroGenics for developing bispecific antibodies. Under the deal, the companies will leverage MacroGenics’ DART platform to develop MGD024 as well as two further bispecific research programmes.
pharmaphorum
DECEMBER 16, 2020
AbbVie is to begin clinical development of an antibody designed to neutralise the SARS-CoV-2 coronavirus after licensing the therapy in from Harbour BioMed and Utrecht University. AbbVie has begun a phase 1 clinical trial of the antibody, with clinical development beginning in the US and expanding into Europe.

BioSpace
OCTOBER 21, 2021
The license and collaboration agreement allows Janssen to use F-star’s proprietary technology in the research, development and commercialization of novel bispecific antibodies.

pharmaphorum
MAY 18, 2022
Brand new UK startup RQ Biotechnology has been thrust into the spotlight after signing a $157 million licensing deal with AstraZeneca for monoclonal antibodies intended to protect vulnerable and immunosuppressed people from COVID-19. ” The post AstraZeneca buys into startup RQ Bio’s COVID antibodies appeared first on. .

Pharmaceutical Technology
DECEMBER 19, 2022
With the latest development, Sanofi will licence a NK cell engager programme that acts on B7H3 from the antibody-based NK cell engager therapeutics (ANKET) platform of Innate. Initially, the companies signed a research collaboration and licensing agreement in 2016 to develop and assess up to two bispecific NK cell engagers.

pharmaphorum
JANUARY 27, 2021
AstraZeneca is playing catch up with Eli Lilly and Regeneron with its antibody therapy for COVID-19, but aims to narrow the gap with the help of decentralised clinical trial specialist Care Access Research. The two antibodies were discovered by Vanderbilt University Medical Center and licensed to AZ in June 2020.
Pharmaceutical Technology
OCTOBER 25, 2022
There are options in the future to possibly apply the worldwide research and development (R&D), manufacturing and marketing expertise of Astellas in gene therapy to AAV gene therapy development programmes of Taysha for genetic ailments of the central nervous system (CNS).

pharmaphorum
JANUARY 7, 2021
The partnership covers up to five oncology targets identified by OBT using its discovery platform, and the UK firm will try to develop antibodies against them. Kite and Gilead will then develop and commercialise therapies based on these targets or antibodies.
BioPharma Reporter
JUNE 1, 2021
Corbus Pharmaceuticals announced the expansion of its portfolio into immuno-oncology through licensing deals with the University of California San Francisco and Panorama Research Inc for two new monoclonal antibodies (mAbs).

Pharmaceutical Technology
JANUARY 6, 2023
Dutch biotechnology company Synaffix and Amgen have entered a licensing agreement for the development of next-generation antibody-drug conjugates (ADCs). For four future programmes, Amgen will also have an option for exercising exclusive licenses for research and commercialisation.

pharmaphorum
SEPTEMBER 2, 2020
The drug, JTX-1811, is a monoclonal antibody designed to selectively deplete immunosuppressive tumour-infiltrating T regulatory (TITR) cells. . When JTX-1811 binds to CCR8, it targets TITR cells for depletion by an enhanced antibody-dependent cellular cytotoxicity mechanism. . In 2016 Celgene signed a $2.6

XTalks
JANUARY 29, 2021
Data from Eli Lilly and Company’s (NYSE: LLY) Phase III clinical trial evaluating its monoclonal antibody drugs bamlanivimab (LY-CoV555) and etesevimab (LY-CoV016) show that combining the two significantly reduced the risk of COVID-19 hospitalizations and death by 70 percent. percent) compared with 36 events (7.0

BioSpace
OCTOBER 21, 2021
Atreca announced it is licensing its preclinical monoclonal antibody for the prevention of malaria to the Gates Medical Research Institute (MRI) for development.

BioPharma Reporter
AUGUST 1, 2023
Renaissance Pharma, a company focused on the development of life changing therapies in pediatric rare disease, has entered into an exclusive license agreement with St. Jude Childrenâs Research Hospital for Hu14.18, a humanised antibody in development by the hospital for the treatment of newly diagnosed high-risk neuroblastoma.

The Pharma Data
JANUARY 11, 2021
a San Diego-based biotechnology company with an array of technology platforms for antibody discovery and optimization, and novel NK and T cell engager generation, today announced licensing of a panel of its anti-SARS-CoV-2 antibody clones to IGM Biosciences for COVID-19 therapy development.
XTalks
APRIL 1, 2025
Related: Niktimvo (Axatilimab) Approved as First-in-Class Therapy for Chronic GVHD More on Itolizumabs Clinical Data Itolizumab is a humanized anti-CD6 monoclonal antibody that targets the CD6-ALCAM pathway involved in T cell activation and trafficking. The risk limits the number and type of patients who receive HSCT.
XTalks
MAY 2, 2022
Lecanemab, the product of Phase IIb clinical research completed by BioArctic and collaborator Eisai, is one therapy making strides this year for Alzheimer’s disease. This year, the monoclonal antibody lecanemab is approaching final stages of investigation as a treatment for targeting the A? Next Stages in Clinical Research.
Pharmaceutical Technology
JANUARY 23, 2023
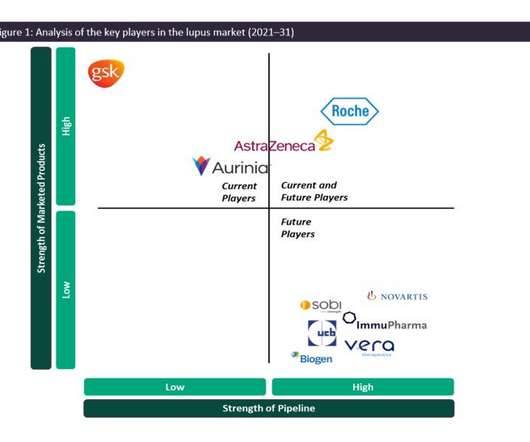
Another key player in the LN market is Aurinia Pharmaceuticals’s Lupkynis, having gained market approval in the US in 2021 and in the EU (licensed to Otsuka Pharmaceutical Co ) in 2022.

The Pharma Data
JUNE 2, 2022
Boehringer Ingelheim and the Agency for Science, Technology and Research (A*STAR) today announced a global licensing agreement under which Boehringer Ingelheim will obtain exclusive worldwide rights to research, develop and commercialize products based on a panel of innovative, tumor-specific antibodies from A*STAR.

Pharmaceutical Technology
OCTOBER 31, 2022
Simultaneously, Genmab has submitted a biologics license application (BLA) to the US Food and Drug Administration (FDA) for the investigational subcutaneous IgG1-bispecific antibody to treat R/R large B-cell lymphoma (LBCL) patients.

XTalks
JANUARY 22, 2025
IXCHIQ is the only licensed chikungunya vaccine in the US for adults aged 18 and older at increased risk of exposure to the virus. seroresponse rate indicating the proportion of participants achieving antibody levels above a predefined protective threshold sustained one year after vaccination. The findings reveal a remarkable 98.3%
The Pharma Data
MARCH 9, 2021
. (“Biolojic”), a biotechnology company that computationally designs functional antibodies, today announced a research collaboration and license agreement that will leverage Biolojic’s AI-based multibody platform to discover and develop a potential novel antibody-based therapy for the treatment of diabetes.

Pharmaceutical Technology
DECEMBER 28, 2022
Gilead Sciences and Jounce Therapeutics have amended their current license agreement for first-in-class immunotherapy, GS-1811 (formerly JTX-1811). The initial license agreement was signed in 2020. Now, the company will be solely responsible for all further research, development, and commercialisation of GS-1811 across the world.
Pharmaceutical Technology
DECEMBER 6, 2022
In the partnership’s discovery phase, RAVEN, an artificial intelligence (AI) platform of Evaxion will be leveraged for designing a next-generation vaccine candidate that induces cellular as well as humoral/antibody responses. Using ExpreS 2 ion’s ExpreS2 platform, the company will manufacture the antigen constructs obtained from RAVEN.

Pharmaceutical Technology
FEBRUARY 3, 2023
Researchers have relentlessly worked through the barriers to create lifechanging treatments for patients in need. “As For example, researchers have developed a two-plasmid cotransfection system. Moving the viral vector field closer to the monoclonal antibody field is really what we see as the way forward.”
Pharmaceutical Technology
AUGUST 11, 2022
Last year, Disc in-licensed bitopertin from Roche. In-licensed from AbbVie in 2019, DISC-0974 is a monoclonal antibody. Disc also has a research programme which can detect orally available small molecules to hinder Matriptase-2 and boost the hepcidin production and hinder iron availability.

Delveinsight
JANUARY 12, 2021
Medivir, IGM Biosciences enters into an exclusive licensing agreement for Birinapant. Medivir AB has entered into an exclusive licensing agreement with IGM Biosciences to receive global, exclusive development rights for Birinapant. AvantGen Enters into a Licensing Agreement for its Anti-SARS-CoV-2 Antibodies with IGM Biosciences.

Pfizer
JANUARY 27, 2023
Pfizer Responds to Research Claims carterda Fri, 01/27/2023 - 19:48 Pfizer Responds to Research Claims Friday, January 27, 2023 - 08:00pm Share New York, N.Y., In the ongoing development of the Pfizer-BioNTech COVID-19 vaccine, Pfizer has not conducted gain of function or directed evolution research.

pharmaphorum
JANUARY 29, 2021
US drugmaker Eli Lilly – still waiting for an FDA decision on one non-opioid pain drug – has just added another to its pipeline via a licensing agreement with Japan’s Asahi Kasei worth up to $410 million. While tanezumab is an antibody and has to be delivered by subcutaneous injection, AK1780 is orally bioavailable.
pharmaphorum
JANUARY 13, 2022
Sanofi has joined a growing list of drugmakers going after alpha-synuclein targeting drugs for Parkinson’s disease, licensing a bispecific antibody from South Korea’s ABL Bio in a deal that could be worth more than $1 billion. Despite the interest, there have already been some failures in the alpha-synuclein category.
Pharmaceutical Technology
APRIL 17, 2023
Telix has announced the successful preclinical development of radiolabelled olaratumab, an antibody licensed by Eli Lilly and Company. Telix has demonstrated proof-of-concept (PoC) and will now progress to first-in-human clinical studies.
Pharmaceutical Technology
NOVEMBER 18, 2022
Regeneron Pharmaceuticals has entered a collaboration and licensing agreement with CytomX Therapeutics for developing conditionally-activated bispecific cancer therapies. Under the deal, the companies will work together on the discovery activities for identifying and validating the conditionally active bispecific antibodies.

XTalks
FEBRUARY 12, 2025
Recent advances highlight a broader wave of innovation in pulmonary fibrosis research. Similarly, Mediar Therapeutics entered a global licensing agreement with Eli Lilly to advance MTX-463, a first-in-class human IgG1 antibody that neutralizes WISP1-mediated fibrotic signaling, into a Phase II trial.

Camargo
DECEMBER 13, 2021
Both the FDA’s Center for Drug Evaluation and Research (CDER) and its Center for Biologics Evaluation and Research (CBER) have regulatory responsibility for therapeutic biological products, which are subject to both the Federal Food, Drug and Cosmetic (FD & C) Act and the Public Health Service (PHS) Act.

pharmaphorum
NOVEMBER 18, 2022
have announced a strategic research collaboration within the field of conditionally activated bispecific therapeutics for the treatment of cancer. Using CytomX’s Probody and Regeneron’s Veloci-Bi platforms, the collaboration and licensing agreement aims to enable the development of investigational next-generation bispecific immunotherapies.

The Pharma Data
DECEMBER 14, 2020
Harbour BioMed (HBM) announced with Utrecht University on Monday that they have licensed their fully human SARS-CoV-2 neutralizing antibody, 47D11, and its program to AbbVie. AbbVie has initiated a Phase I clinical trial of the antibody, and it will conduct the initial clinical program in the U.S.
Pharmaceutical Technology
MAY 24, 2023
Danyelza is a recombinant humanised monoclonal antibody that acts on the ganglioside GD2 that is highly expressed in several neuroectoderm-derived tumours and sarcomas. It was developed by researchers at the Memorial Sloan Kettering Cancer Center in New York and is exclusively licensed to Y-mAbs.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?



Let's personalize your content