Bristol Myers pours $100M into RNA drugs for cardiovascular diseases
Bio Pharma Dive
NOVEMBER 28, 2023
Tuesday’s deal signals the pharmaceutical giant's confidence in Avidity Biosciences and its so-called antibody oligonucleotide conjugates.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Bio Pharma Dive
NOVEMBER 28, 2023
Tuesday’s deal signals the pharmaceutical giant's confidence in Avidity Biosciences and its so-called antibody oligonucleotide conjugates.
Pharmaceutical Technology
JULY 15, 2022
Health Canada has granted approval for the usage of Moderna’s messenger RNA (mRNA) Covid-19 vaccine, Spikevax, in a 25µg two-dose regimen for active immunisation to prevent Covid-19 in children aged six months to five years. So far, children aged below five years were not eligible to receive the Covid-19 vaccine in Canada.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

pharmaphorum
MARCH 3, 2024
Avidity Biosciences files $400m private placement as it prepares for phase 3 trial of lead antibody oligonucleotide conjugate AOC 1001 in rare disease DM1

pharmaphorum
MAY 12, 2021
UK biotech MiNA Therapeutics has signed up another big pharma partner for its small activating RNA (saRNA) platform, which upregulates the activity of proteins, with Eli Lilly the latest to get in on the action. . The post Lilly buys into MiNA’s protein-boosting RNA tech in $1.25bn deal appeared first on.
Pharmaceutical Technology
JANUARY 19, 2023
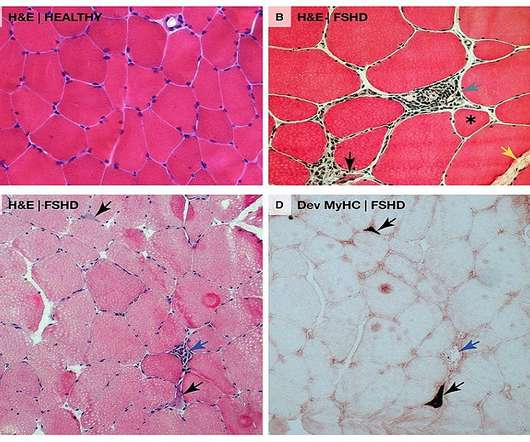
It comprises a monoclonal antibody (mAb) attached to the transferrin receptor 1 (TfR1) conjugated with a DUX4 mRNA that targets siRNA. We look forward to working collaboratively with the FDA to bring the first RNA therapy directly targeting DUX4 to patients as quickly as possible.”

Scienmag
FEBRUARY 7, 2022
It folds the RNA of the virus into a compact structure. COVID-19 patients usually develop antibodies (immunity proteins that are specifically […]. The SARS-CoV-2 nucleoprotein is the main protein in viral particles.

Pharmaceutical Technology
DECEMBER 21, 2023
A patent has been filed for an intravenous administration of an anti-transferrin receptor antibody linked to an oligonucleotide, targeting the degradation of DUX4 mRNA and biomarker RNA in muscle cells. Discover a groundbreaking method for treating Facioscapulohumeral muscular dystrophy (FSHD) and other muscular dystrophies.
XTalks
JANUARY 21, 2025
The liquid portion of the blood (serum or plasma) containing antibodies is separated and tested using the Liason Biotrin parvovirus B19 IgG plus test on a Liason XL analyzer. If parvovirus B19 antibodies are present, they bind to the chemicals and particular particles in the test, producing a light signal.

Pharmaceutical Technology
OCTOBER 5, 2022
miRecule and Sanofi have entered a strategic partnership and exclusive licence agreement for the development and commercialisation of an antibody-RNA conjugate (ARC) to treat facioscapulohumeral muscular dystrophy (FSHD). The anti-DUX4 RNA therapy was discovered using the DREAmiR platform of miRecule.

pharmaphorum
OCTOBER 5, 2022
Sanofi has added to its rare disease pipeline by licensing an antibody-RNA conjugate (ARC) for facioscapulohumeral muscular dystrophy (FSHD), a genetic muscle disorder, from US biotech miRecule. billion deal in 2018.

BioSpace
JANUARY 20, 2021
California-based Avidity Biosciences has a goal of disrupting the way RNA-based therapies are delivered to patients through its Antibody-Oligonucleotide Conjugates (AOCs) platform.

Roots Analysis
OCTOBER 30, 2023
In the past few years, Next Generation RNA therapeutics have emerged as one of the key therapeutic modalities in the modern healthcare industry. These RNA based therapeutics play a crucial role in protein production and regulation of gene functions.
Pharmaceutical Technology
AUGUST 24, 2022
Mode rna has submitted an application to the US Food and Drug Administration (FDA) to obtain emergency use authorization (EUA) for mRNA-1273.222, its BA.4/BA.5 As against a 50µg booster dose of mRNA-1273 in priorly uninfected subjects, mRNA-1273.214 demonstrated superior neutralising antibody response against Omicron BA.1 1 subvariant.

Medical Xpress
DECEMBER 13, 2022
Molecular tests measure the presence of viral SARS-CoV-2 RNA while serology tests detect the presence of antibodies triggered by the SARS-CoV-2 virus. Serology (blood) and molecular tests are the two most commonly used methods for rapid COVID-19 testing. Because COVID-19 tests use different mechanisms, they vary significantly.

The Pharma Data
OCTOBER 30, 2020
Neutralizing Antibody Shows Potential for Mild-to-Moderate COVID-19. 30, 2020 — Viral load was decreased at day 11 for patients with mild or moderate COVID-19 who received a single intravenous infusion of virus-neutralizing monoclonal antibody versus placebo, according to a study published online Oct. Professional. FRIDAY, Oct.
XTalks
OCTOBER 7, 2020
While the new test is based on RT-PCR molecular detection, it utilizes a high-throughput technique involving a new heat-based extraction method and enhanced technology to extract RNA from samples for COVID-19 testing. LabCorp was given the green light for the test by the FDA last week, which is set to be rolled out soon.

BioPharma Reporter
APRIL 24, 2023
Avidity Biosciences, a biopharmaceutical company developing a new class of RNA therapeutics called Antibody Oligonucleotide Conjugates (AOCs), today announced that the US Food and Drug Administration (FDA) has granted Fast Track designation to AOC 1044.

pharmaphorum
JANUARY 7, 2022
NI006 is an antibody that binds to the misfolded forms of the transthyretin protein that form the fibrils, and cause them to be cleared away by immune cells like macrophages. billion on the first nine months of 2021, well ahead of Pfizer’s own forecasts. Tafamidis is thought to work by stabilising the misfolded proteins.

Delveinsight
AUGUST 26, 2021
Shape’s RNA editing technologies can modify the RNA sequence, which makes the body’s protein building blocks. This system is designed to deliver RNA editing technology or other payloads directly to particular body areas, such as the nervous system or muscle. The study is set to conclude in the fall.

XTalks
FEBRUARY 12, 2025
Current PCSK9 inhibitors on the market include Regenerons monoclonal antibody Praluent (alirocumab), Amgens Repatha (evolocumab) and Novartiss Leqvio (inclisiran), which is a small RNA interference (siRNA) therapy designed to lower LDL-C. This gives lerodalcibep an edge to approved PCSK9 inhibitors.
Pharmaceutical Technology
JULY 5, 2022
On leaving the liver cells, the virus hijacks bits of cell membrane and conceals itself from antibodies that would have isolated the virus before it travelled far through the bloodstream. In addition, the scientists later found that the HAV needs TENT4A/B for its replication.
Pharmaceutical Technology
FEBRUARY 15, 2023
Adeno-associated virus vectors, alcohol dehydrogenase compositions, and antibody serum stabilisers are some of the accelerating innovation areas, where adoption has been steadily increasing. Among maturing innovation areas are anti-influenza antibody compositions and anti-interleukin-1, which are now well established in the industry.

pharmaphorum
JULY 6, 2021
The small interfering RNA (siRNA) therapy has already been approved in Europe as Leqvio , and the FDA’s rejection was not caused by any issues with its efficacy or safety, according to Novartis.

pharmaphorum
SEPTEMBER 16, 2020
Both of these vaccines are based on RNA and in phase 3 and phase 1 testing, respectively. Earlier clinical data has shown that the vaccine can stimulate neutralising antibodies against SARS-CoV-2 and was well-tolerated with a transient mild to moderate fever being the main side effect seen in less than 20% of recipients.
pharmaphorum
DECEMBER 1, 2020
The EMA has also confirmed it has received a filing from Moderna, which is producing a rival vaccine based on similar RNA technology. If approved these vaccines will break new ground as it will be the first time RNA vaccines have been used.
Pharmaceutical Technology
JUNE 8, 2023
The company’s samRNA vector is based on a synthetic RNA molecule derived from a wild-type Venezuelan Equine Encephalitis Virus (VEEV) replicon with the goal of extending the duration and magnitude of immunogen expression to drive potent and durable immune responses.

pharmaphorum
DECEMBER 23, 2021
The first-in-class, small interfering RNA (siRNA) therapy for cholesterol lowering inhibiting PCSSK9 – the same target as Amgen’s Repatha (evolocumab) and Sanofi/Regeneron’s Praluent (alirocumab) – but after a lead-in period is dosed only twice a year rather than every month.

XTalks
APRIL 22, 2022
Generally, antibody-based therapeutics are one of the main types of drugs that are assessed using receptor occupancy assays as they bind to specific cell surface receptors through which they impact downstream intracellular signalling pathways. The antibodies are conjugated to fluorochromes for them to be detected.
The Pharma Data
OCTOBER 25, 2020
The messenger RNA-based vaccine, which is designed to block replication of the coronavirus, was shown to induce neutralizing antibodies and activate T-cells in hamsters and mice. German drugmaker CureVac said preclinical studies of its COVID-19 vaccine candidate, CVnCoV, showed the vaccination prompted a successful immune response.
pharmaphorum
AUGUST 18, 2020
The consortium aims to rapidly develop drugs to fight SARS-CoV-2 and find virus neutralising antibodies. Hallett said that the targets being explored are the proteins involved in viral replication – the protease, the RNA polymerase, the papain-lie protease and elements of the “Spike” protein that allows the virus to infect cells.

The Pharma Data
SEPTEMBER 10, 2020
According to the data, the vaccine candidate conferred “protective anti-viral effects” in rhesus macaque monkeys, as well as concomitant high neutralising antibody titers and a TH1-biased cellular response in both the monkeys and in mice.
XTalks
JULY 14, 2022
Over time, even the use of Krystexxa can lead to the development of antibodies against it, reducing its effectiveness. Early data also showed that methotrexate could prevent the formation of anti-drug antibodies. This is why methotrexate was paired with it, as methotrexate is used to treat inflammatory conditions like arthritis.

The Pharma Data
JANUARY 20, 2021
based Gritstone Oncology is developing a novel COVID-19 vaccine based on adenovirus and messenger-RNA technology designed to combat future variants of the coronavirus. Emeryville, Calif.-based Louis University. . Source link.
pharmaphorum
NOVEMBER 17, 2020
Both Pfizer and Moderna vaccines use synthetic messenger RNA to activate the immune system against the virus. They both code for the “Spike” protein seen on the surface of the SARS-CoV-2 coronavirus, which causes the body to produce antibodies that neutralise the virus in the event of an infection.

Pharmaceutical Technology
APRIL 24, 2023
Since then, the field of nanomedicine has steadily progressed to reach high points such as the successful use of nanotechnology to deliver messenger RNA (mRNA)-based Covid-19 vaccines. This design protects antibodies from the innate immune system. And the issue is that antibodies are too big to go directly inside the cell,” he says.

XTalks
FEBRUARY 3, 2022
The anti-amyloid antibody will go head-to-head against Biogen’s Aduhelm (aducanumab), which continues to face controversy over its approval given questions about its lacklustre clinical benefit. Related: Eli Lilly Pursues RNA Editing in New Partnership with ProQR. Last year, Lilly announced a new, $1.5

pharmaphorum
MARCH 1, 2021
COVID-19 antigen tests diagnose active infection by detecting SARS-CoV-2 viral proteins in samples, as opposed to PCR tests which detect viral RNA and require lab processing, but can miss infections with low virus levels.

pharmaphorum
OCTOBER 6, 2020
These studies suggest BNT162b2 triggers the production of neutralising antibodies and T-cells that target SARS-CoV-2, the coronavirus that causes COVID-19.

The Pharma Data
AUGUST 16, 2021
Better activation of innate and adaptive immune responses was achieved with CV2CoV, resulting in faster response onset, higher titers of antibodies, and stronger memory B and T cell activation as compared to the first-generation candidate, CVnCoV. The full manuscript of the preclinical data is available on the pre-print server bioRxiv. “In

The Pharma Data
DECEMBER 8, 2020
Since partnering with Eli Lilly to produce the first monoclonal antibody therapy approved for mild-to-moderate COVID-19 patients, antibody discovery company AbCellera is going big on the Nasdaq. Laying down a new track for RNA processing, Remix launched with $81 million in financing. Reduce cost. Nuance Pharma .
pharmaphorum
MARCH 9, 2022
Sanofi and Sobi’s haemophilia partnership has been under competitive pressure from new therapies like Roche’s antibody Hemlibra, so the two companies are hoping a new long-acting drug candidate can revitalise the franchise.

pharmaphorum
JULY 13, 2022
The first to reach the market were antibodies – Amgen’s Repatha (evolocumab) and Sanofi/Regeneron’s Praluent (alirocumab) – which reached the market several years ago. If it makes it through development, VERVE-101 could provide a one-off alternative to a lengthening list of PCSK9 inhibitors that have to be dosed chronically.

BioSpace
NOVEMBER 20, 2022
Former Theranos CEO Elizabeth Holmes will serve 11 years in prison on four counts of fraud, according to her sentencing by a federal judge in a California courtroom on Friday afternoon.

pharmaphorum
APRIL 5, 2022
.” Strong efficacy data is likely to be the key to carving out a role for the antisense drug in the PCSK9 inhibitor class, particularly as it is up against some heavyweight competition – notably Novartis’ small interfering RNA (siRNA) therapy Leqvio (inclisiran), which only needs to be administered twice a year.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content