Drug Excipient Business Development Reports now Available on Amazon
Drug Patent Watch
FEBRUARY 15, 2024
Elevate your excipient business strategies with the comprehensive Drug Excipient Business Development Reports from DrugPatentWatch.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Drug Patent Watch
FEBRUARY 15, 2024
Elevate your excipient business strategies with the comprehensive Drug Excipient Business Development Reports from DrugPatentWatch.
Bio Pharma Dive
SEPTEMBER 26, 2023
Much anticipated clinical trial data for Immunovant’s experimental FcRn inhibitor could catalyze business development decisions for its parent, Roivant Sciences.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
OCTOBER 2, 2023
The divestments will bring in more than $3 billion as the company works to reduce its debt and plan for future business development.

Pharmaceutical Technology
APRIL 20, 2023
Chinese firm Hasten Biopharmaceutic has raised $315m for business development of its pipeline assets in a financing round co-led by Singapore-based CBC Group and Abu Dhabi sovereign wealth fund Mubadala Investment, The funding round was supported by additional institutional investors.

Pharmaceutical Technology
APRIL 26, 2023
This investment will be used to further the development of the Singapore-based pharmaceutical company’s solid tumour-focused monoclonal antibodies. The funding and a prize-win from Amgen will support the company’s drug development plans as Singapore continues developing its pharmaceutical market.

Pharmaceutical Technology
AUGUST 30, 2022
Zerion Pharma has entered a partnership with Insud Pharma for the development and marketing of drug products using Zerion Pharma's solubility-boosting Dispersome technology. . Under the deal, Zerion will handle the development of Dispersome formulations of marketed drugs.

Drug Patent Watch
DECEMBER 3, 2019
Changes in drug markets can create growth opportunities for API manufacturers and CDMOs alike. Whether change comes from generic entry, from new formulations of drugs, or from competition from novel…. The post Customer Success: API and CDMO Business Development appeared first on DrugPatentWatch - Make Better Decisions.

Drug Patent Watch
DECEMBER 10, 2024
Ensuring Regulatory Compliance CDMOs have extensive knowledge of regulatory requirements and guidelines, which is critical for ensuring compliance with regulatory agencies such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA). CDMOs and How They Support Business Development. References: Ellis, J.
Pharmaceutical Technology
JULY 14, 2022
The commercial investment required to research and develop an innovative drug, prove its safety and efficacy, and bring it to market is staggering. Most famously, the US passed its Orphan Drug Act in 1983, providing innovators with financial motivation to develop orphan drugs and meet the needs of these forgotten patients.

Pharmaceutical Technology
OCTOBER 28, 2022
PTC will use the network and life sciences capabilities of Blackstone to expand its business development works. PTC Therapeutics chief financial officer Emily Hill said: “This strategic financing will support the acceleration of PTC’s robust and diversified pipeline, business development opportunities and general corporate purposes.

Pharmaceutical Technology
JULY 29, 2022
In preparation for this, drug manufacturing will begin later this year and will be outsourced to external contract manufacturing organisations (CMOs). The most common marketed drugs in this space aim to address the hyperammonaemia caused by the defective genes in this disorder. David Bumcrot PhD, CSO, CAMP4 Therapeutics.

Camargo
SEPTEMBER 23, 2020
Panel Summary from the World Orphan Drug Congress 2020. Last month, executives from leading international biopharma companies came together at the World Orphan Drug Congress 2020 to discuss how they are evaluating new opportunities in the rare disease space. Thomas Kassberg, Chief Business Officer, Ultragenyx.

Pharmaceutical Technology
MAY 11, 2008
Pharmaceutical companies, regulatory agencies and governments are becoming increasingly concerned about fraud and counterfeiting throughout the pharmaceutical supply chain, especially with the cost of drugs going up. RFID has other advantages in that it can be used to monitor conditions such as temperature during transport of the drugs.
Pharmaceutical Technology
APRIL 3, 2023
Innate Pharma has signed an exclusive licence agreement with Takeda to enable research and development of antibody drug conjugates (ADCs) against an undisclosed target, primarily focusing on coeliac disease. In addition, Innate will receive royalties on possible net sales of any commercial product to be developed under the licence.
Pharmaceutical Technology
JULY 1, 2022
The funding will offer capital to extend Blueprint’s varied pipeline toward marketing as well as to continue seeking strategic and synergistic business development prospects. Ayvakit has received the US Food and Drug Administration (FDA) approval to treat advanced systemic mastocytosis in adults.
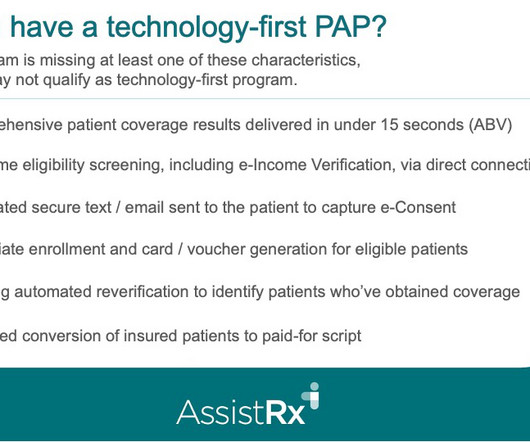
Drug Channels
MARCH 10, 2023
Today’s guest post comes from Stacey Little, Senior Vice President of Business Development and Marketing at AssistRx. Download AssistRx's free 61-page eBook here: Specialty Drug Patient Support Programs: 2022 Progress Report. d/b/a Drug Channels Institute. Read on for Stacey’s insights.

Drug Channels
MARCH 11, 2022
Today’s guest post comes from Stacey Little, Senior Vice President of Business Development and Marketing at AssistRx. d/b/a Drug Channels Institute. Drug Channels® is a registered trademark of Pembroke Consulting, Inc. Stacey analyzes trends in patient support services and reimbursement hubs.

Drug Channels
AUGUST 18, 2023
Today’s guest post is from Divya Iyer, Vice President of Strategy & Business Development, Pharma Manufacturer Solutions, at GoodRx. d/b/a Drug Channels Institute. Drug Channels® is a registered trademark of Pembroke Consulting, Inc. Read on for Divya’s insights. This Feed is for personal non-commercial use only.

Drug Channels
FEBRUARY 21, 2025
Vice President, Business Development at PHIL. Read more 2006-2025 HMP Omnimedia, LLC d/b/a Drug Channels Institute , an HMP Global Company. Today's guest post comes from Thomas Luby, Sr. He lists the key performance indicators that can help brands improve GTN. Read on for Thomas's insights. All rights reserved.

Drug Channels
AUGUST 12, 2022
Today’s guest post comes from Divya Iyer, VP, Strategy and Business Development, Pharma Manufacturer Solutions at GoodRx. d/b/a Drug Channels Institute. Drug Channels® is a registered trademark of Pembroke Consulting, Inc. Read on for Divya’s insights. Read more » Copyright © 2006-2022 Pembroke Consulting, Inc.

Pharmaceutical Technology
FEBRUARY 16, 2023
Under the deal, Aqilion will receive $10.6m (€10m) in upfront cash payment from Merck and also eligible for up to $1,015m (€950m) in potential development and commercialisation milestone payments, along with tiered royalties on global net sales.

The Pharma Data
DECEMBER 13, 2020
.–( BUSINESS WIRE )– EdiGene, Inc. which develops genome editing technologies to accelerate drug discovery and develop novel therapeutics for a broad range of diseases, today announced the appointment of Bo Zhang, Ph.D., as Head of Business Development. He received his B.S.

Pharmaceutical Technology
SEPTEMBER 5, 2022
Wholesale distributors are responsible for guaranteeing product quality and preventing the influx of counterfeited drugs. Wholesalers can be either full-line wholesalers, who purchase the complete product line of a company, or specialised companies, which purchase speciality drugs to sell to clinics and hospitals. GDP and GMP advisory.

pharmaphorum
NOVEMBER 18, 2021
Its vast experience in diabetes and the amount of data collected has propelled Novo Nordisk into exploring new modalities and searching for valuable collaborators, according to the company’s senior VP of global drug discovery Karin Conde-Knape. Karin Conde-Knape is senior VP of global drug discovery, Novo Nordisk.

Pharmaceutical Technology
MAY 11, 2023
Aviko CEO and Deerfield business development vice-president David Greenwald stated: “In spite of improvements in patient outcomes over the past several decades, there is still a significant need for cancer therapies that effectively target cancer cells while leaving healthy tissue unharmed.

Pharmaceutical Technology
SEPTEMBER 5, 2022
The information contained within the download document is designed for pharmaceutical executives, marketing managers, product managers, sales and marketing executives, medical representatives, software engineers, developers, business development executives and any other individual involved in the direct-to-consumer pharmaceutical marketing industry.

Pharmaceutical Technology
FEBRUARY 22, 2023
The deal terms include an upfront fee along with several development, regulatory, and commercial milestone payments. The US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) granted orphan drug designations to ASN90 for the treatment of PSP.

XTalks
DECEMBER 12, 2024
The program comes as the pharmaceutical giant continues to build on its remarkable growth trajectory driven by blockbuster GLP-1 diabetes and obesity drugs Mounjaro and Zepbound, respectively. billion to business development, $3.5 Mounjaro generated $3.11 billion in sales while Zepbound revenues totaled $1.26

Drug Channels
AUGUST 13, 2021
Today’s guest post comes from Stacey Little, Senior Vice President of Business Development and Marketing at AssistRx. d/b/a Drug Channels Institute. Drug Channels® is a registered trademark of Pembroke Consulting, Inc. She offers three criteria that manufacturers can use to evaluate e-support services providers.

Pharmaceutical Technology
SEPTEMBER 7, 2022
The list includes companies offering various products and services, including but not limited to: · Over-the-counter drugs. Prescription refill and generic drug programmes. Speciality care. Compounding services. Pharmacogenetics testing. Filling services. Proprietary pharmaceutical, nutraceutical, and cosmeceutical products.

pharmaphorum
JANUARY 24, 2022
Exploring the Impact of Machine Learning and Artificial Intelligence in Drug Development from Discovery to Healthcare. SMi Group is proud to present its 3rd Annual AI in Drug Discovery Conference , taking place on the 14th and 15th March 2022 in London, UK. Chaired by: Darren Green, Director of Computational Chemistry, GSK.

Pharmaceutical Technology
SEPTEMBER 14, 2022
The growing demand for innovative drugs and a limited number of blockbuster drugs has led to the growth of the pharmaceutical contract manufacturing market, which has fuelled contract commercial dose manufacturing and marketing.

pharmaphorum
MAY 18, 2022
Merck & Co has quietly added another drug to its immuno-oncology pipeline via an agreement with China’s Sichuan Kelun Pharmaceutical, and is keeping the details close to its chest. A total of $17 million of the upfront payment has already been paid by Merck as part of an earlier collaboration on the drug.

Pharmaceutical Technology
SEPTEMBER 15, 2022
Various factors have contributed to the need and growth of API chemical suppliers such as rising healthcare expenditure, increasing disposable incomes, growing geriatric population, increasing incidence of chronic diseases, patent expiration of blockbuster drugs, increased consumption of generic drugs, and intervention of the new generation APIs.

pharmaphorum
NOVEMBER 7, 2022
Prior to joining 1910 Genetics, Genestin was vice president of business development and pipeline strategy at Hikma Pharmaceuticals and member of the Specialty & Generics leadership team. . “I am excited to join 1910 Genetics at such a pivotal time for the company,” Genestin said.

The Pharma Data
JANUARY 5, 2021
The funds will help advance one of its drug candidates, CPV-101, to stages that will facilitate further investment from venture capitalists and/or pharmaceutical companies. Eleva has been utilizing its unique moss-based platform to produce novel drugs. Ralf Smit as CBO (Chief Business Officer) to its Executive Board.

Pharmaceutical Technology
APRIL 26, 2023
Today’s digital inhaler space requires “consistent engagement over an extended timeframe,” says Francis White, global vice president of business development at Adherium , along with “showing commitment while continuing to prove the patient benefits, cost reductions, and reduction of staff burden.”

Pharmaceutical Technology
SEPTEMBER 14, 2022
API peptides and proteins-based drugs have gained much attention in the past decade. Victoza, Copaxone, Lupron, Zoladex, Sandostatin, and Somatuline are some of the popularly marketed peptide API therapeutic drugs while more than 600 peptide-based pharmacological leads are being investigated worldwide, across various phases of development.

pharmaphorum
SEPTEMBER 27, 2022
While a rumoured takeover by Merck & Co has yet to materialise, Seagen is getting on with its own business development, including a just-agreed licensing deal for a cancer immunotherapy developed by Dutch biotech Lava Therapeutics. 2 T cells, which have potent tumour-killing activity.

Pharmaceutical Technology
SEPTEMBER 8, 2022
Increasing drug failure rates and essential drug shortages have driven the growth of the compounding pharmacies market, which has a high growth potential, especially in emerging economies. Test method development. It may provide access to discontinued drugs and allows for alternative compounded dosage forms.

Pharmaceutical Technology
SEPTEMBER 8, 2022
Pharmaceutical solid dosage forms are the most popular in the pharmaceutical industry and are one of the most used drug delivery methods across patient groups. Drug manufacturers have been investing significantly in the R&D of more effective and patient-friendly solid forms.

BioPharma Reporter
MAY 15, 2024
Before the industry is set to arrive in San Diego for Bio International 2024, we spoke with Dr. Harald Oberegger, global head business development at Novartis Contract Manufacturing.

Pharmaceutical Technology
SEPTEMBER 15, 2022
Research by GlobalData (Contract Small Molecule API Manufacturing Industry by the Numbers – 2021 Edition) shows that containment facilities are in high demand as the use of cytotoxic drugs continues to grow. Adding to these factors is that oncology accounts for the majority of marketed, contained drugs.

Drug Channels
SEPTEMBER 18, 2020
Today’s guest post comes from Ela Lourido, Vice President of Business Development at Biologics by Mckesson. d/b/a Drug Channels Institute. Drug Channels® is a registered trademark of Pembroke Consulting, Inc. Read on for Ela’s insights. Read more » Copyright © 2006-2020 Pembroke Consulting, Inc.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content