Grand Rounds December 15, 2023: Diversifying Clinical Trials: A Path Forward (Roxana Mehran, MD, FACC, FAHA, MSCAI, FESC)
Rethinking Clinical Trials
DECEMBER 20, 2023
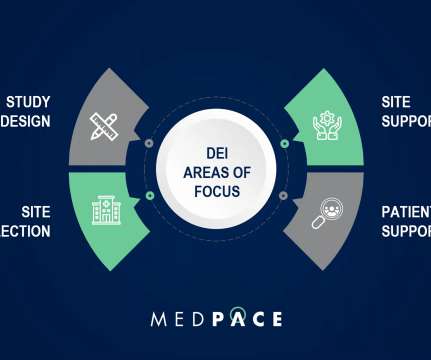
Yet enrollment of ethnic minorities in NIH clinical trials and for trials studying approved devices and drugs remains low. Further efforts are urgently needed to increase diversity in the cardiology workforce, which will improve clinical trial diversity and cardiovascular health for all.













































Let's personalize your content