Cardiovascular Clinical Trials with Patient Diversity: Challenges and Considerations
XTalks
AUGUST 4, 2022
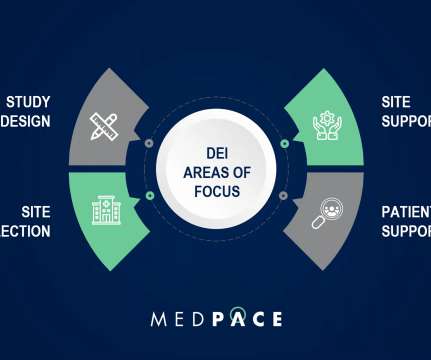
This started back in 1993 when the FDA established guidelines to increase diversity by gender and race/ethnicity of participants in clinical trials that were contributing to new drug approvals. Why Should We Care About Diversity and Equity in Clinical Research? In the same webinar, Dr. Hansie M.








Let's personalize your content