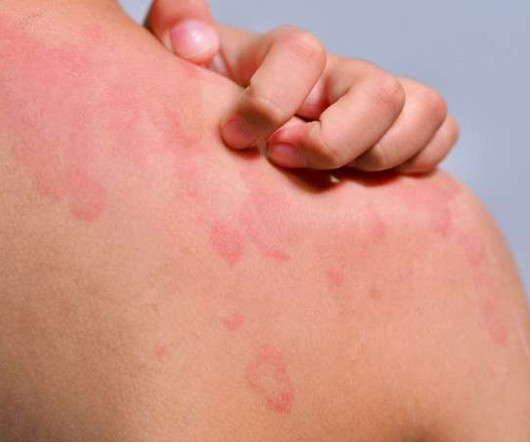
Clinical trial investigates oral MRGPRX2 antagonist in urticaria
Drug Discovery World
SEPTEMBER 4, 2024
Evommune’s pre-clinical data demonstrates that by blocking activation of MRGPRX2 via all disease relevant ligand categories, EVO756 can prevent the degranulation of mast cells. “As we continue to execute on our clinical development plans for EVO756, we are commencing this Phase II trial in 15 study sites across the United States.








































Let's personalize your content