Study proves efficacy of novel protein conjugate in autoimmune disease
Drug Discovery World
JUNE 10, 2024
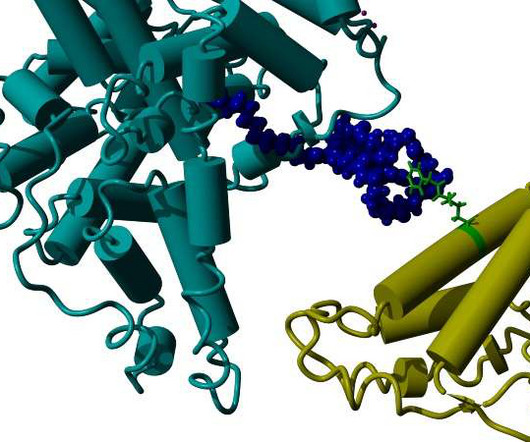
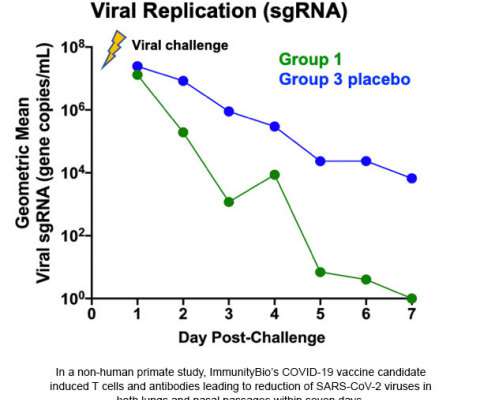
VALANX Biotech has reported successful in vivo results in its lead programme VLX101 for the treatment of a range of autoimmune diseases. Protein drugs hold great promise as therapeutics for a wide range of diseases, however, one of the greatest challenges is their short circulation half-life.






















Let's personalize your content