BioMarin pares Roctavian spending as it narrows sales focus
Bio Pharma Dive
AUGUST 6, 2024
The company plans to limit sales of the hemophilia gene therapy to the U.S., Italy and Germany, while ending most clinical development work.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Bio Pharma Dive
AUGUST 6, 2024
The company plans to limit sales of the hemophilia gene therapy to the U.S., Italy and Germany, while ending most clinical development work.

Pharmaceutical Technology
OCTOBER 21, 2022
Hookipa Pharma and Roche have signed a strategic partnership and licence agreement for developing HB-700 and another undisclosed arenaviral immunotherapy. Under the deal, Hookipa will carry out research and initial clinical development through Phase Ib for HB-700 to treat KRAS-mutated cancers.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
JULY 12, 2022
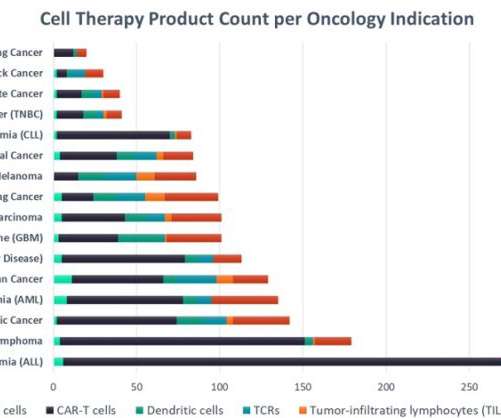
The leading marketed CAR-T cell products, Gilead’s Yescarta (axicabtagene ciloleucel) and Novartis’ Kymriah (tisagenlecleucel), achieved $695m and $587m in sales respectively last year, demonstrating the high potential for sales of successful new cell therapies.

Pharmaceutical Technology
JUNE 12, 2023
Accordingly, the development of non-opioid painkillers is experiencing a surge in activity and despite numerous novel targets receiving high levels of attention, cannabinoids have emerged as strong favourites to replace opioid-related medications. On 9 May, National Fentanyl Awareness Day was observed in recognition of the US opioid crisis.

Pharmaceutical Technology
DECEMBER 14, 2022
GSK has entered a strategic partnership with Wave Life Sciences to progress the discovery and development of oligonucleotide therapies for new genetic targets. The discovery alliance, with a preliminary research period of four years, will aid GSK in progressing up to eight programmes and Wave to develop up to three programmes.
Pharmaceutical Technology
AUGUST 4, 2022
Poseida Therapeutics and Roche have signed a strategic partnership and licence agreement to develop allogeneic CAR-T cell therapies for hematologic malignancies. Roche and Poseida will also partner in a research programme for creating and developing next-generation features and enhancements for allogeneic CAR-T therapies.

Pharmaceutical Technology
OCTOBER 18, 2022
Gilead Sciences has entered an exclusive option and partnership agreement with MacroGenics for developing bispecific antibodies. Under the deal, the companies will leverage MacroGenics’ DART platform to develop MGD024 as well as two further bispecific research programmes.

Pharmaceutical Technology
AUGUST 30, 2022
Zerion Pharma has entered a partnership with Insud Pharma for the development and marketing of drug products using Zerion Pharma's solubility-boosting Dispersome technology. . Under the deal, Zerion will handle the development of Dispersome formulations of marketed drugs.

Pharmaceutical Technology
NOVEMBER 21, 2022
A clinical-stage biopharmaceutical firm, Imago focuses on the development of new therapies to treat myeloproliferative neoplasms (MPNs) and other bone marrow ailments. In October this year, Merck reported worldwide sales of $15bn in the third quarter (Q3) of 2022 as against $13.15bn in the year-ago quarter, indicating a rise of 14%.
Pharmaceutical Technology
NOVEMBER 18, 2022
Regeneron Pharmaceuticals has entered a collaboration and licensing agreement with CytomX Therapeutics for developing conditionally-activated bispecific cancer therapies. This collaboration will use Regeneron’s Veloci-Bi bispecific antibody development platform and CytomX's Probody therapeutic platform.

pharmaphorum
MARCH 14, 2022
This morning, Sandoz announced it has acquired medical and drug delivery device specialist Coalesce Product Development, bolstering its capabilities for the development of respiratory medicines and ‘complex’ generics. The post Sandoz bulks up ahead of possible sale with Coalesce buy appeared first on.

Pharmaceutical Technology
DECEMBER 7, 2022
Pfizer has entered a research partnership and exclusive licence agreement with Clear Creek Bio to progress new SARS-CoV-2 papain-like protease (PLpro) inhibitors’ discovery and development for the oral treatment of Covid-19. On entering the clinic, Pfizer will solely handle the development and marketing works of the candidate.
Pharmaceutical Technology
AUGUST 24, 2022
Lacerta Therapeutics has entered a new licensing and research partnership agreement with Eli Lilly and Company subsidiary, Prevail Therapeutics, to discover and develop adeno-associated virus (AAV) capsids for treating central nervous system (CNS) diseases. . Topic sponsors are not involved in the creation of editorial content.

Pharmaceutical Technology
APRIL 19, 2023
Camlipixant is being assessed in the CALM Phase III clinical development programme, with anticipated regulatory approval and launch in 2026. According to current clinical data, the therapy may reduce cough frequency for RCC patients with a relatively low incidence of dysgeusia by selectively inhibiting P2X3 receptors.
Pharmaceutical Technology
MAY 31, 2023
C4 Therapeutics has entered an exclusive licensing agreement with Betta Pharmaceuticals to develop and commercialise CFT8919 across the greater China region. It is expected to accelerate the development of CFT8919 in important international markets.
Pharmaceutical Technology
APRIL 25, 2023
3BP receives an initial payment of $40m, and $425m as development, regulatory and commercial milestone payments. Tiered royalties on net sales are also included in the deal. 3BP continues to hold specific rights to develop its FAP-targeting imaging technology for diagnostic applications.

XTalks
MARCH 17, 2025
Roche, a global leader in biotechnology and diagnostics, has entered into an exclusive collaboration and licensing agreement with Zealand Pharma, a specialist in innovative therapeutics, to co-develop and co-commercialize petrelintide as a potential foundational therapy for people with overweight and obesity. billion $1.4

Pharmaceutical Technology
FEBRUARY 14, 2023
Precision oncology firm Corbus Pharmaceuticals and CSPC Megalith Biopharmaceutical have entered an exclusive licensing agreement for the latter’s new clinical stage antibody drug conjugate (ADC), CRB-701 (SYS6002). upfront payment and eligible for royalties on net sales. CSPC Megalith is a subsidiary of CSPC Pharmaceutical Group.

Pharmaceutical Technology
JANUARY 16, 2023
Ono Pharmaceutical and Monash University have entered an option and research collaboration for the discovery and development of antibodies that target G protein-coupled receptors (GPCRs). The collaboration is focused on developing anti-GPCR antibodies, which enable the creation of new treatments for autoimmune and inflammatory diseases.

Pharmaceutical Technology
DECEMBER 7, 2022
The partnership will utilise the Smart Allostery drug discovery platform of HotSpot to develop the first and only small molecule inhibitor of IRF5 for these diseases. HotSpot is also entitled to get option fees and research and development milestone payments totalling up to $295m.

pharmaphorum
NOVEMBER 17, 2020
Lead Pharma has signed a potential 260 million euro ($308 million) deal with Roche to develop small molecule drugs for immune diseases. Lead will lead the selection of a pre-clinical drug candidate, after which Roche will be responsible for further development and global marketing. The US biotech got $42.5

Pharmaceutical Technology
AUGUST 17, 2022
Merck (MSD outside North America) has entered a partnership agreement with Orna Therapeutics for discovering, developing and marketing various programmes based on next-generation RNA technology. Merck will also make royalty payments on any approved products developed out of the partnership.
pharmaphorum
DECEMBER 16, 2020
AbbVie is to begin clinical development of an antibody designed to neutralise the SARS-CoV-2 coronavirus after licensing the therapy in from Harbour BioMed and Utrecht University. AbbVie has begun a phase 1 clinical trial of the antibody, with clinical development beginning in the US and expanding into Europe.

Pharmaceutical Technology
JUNE 2, 2023
Amarin research and development president and chief scientific officer Dr Steven Ketchum said: “We congratulate our partner, EDDING, on the regulatory approval of Vascepa in Mainland China, as this marks an important step in the process of offering this novel treatment to patients across that country.”

pharmaphorum
MAY 4, 2021
The agreement allows for a clinical development programme for Vicore’s digital therapeutic (DTx) VP04. million Swedish kroner (around $1 million) plus potential milestone payments and royalties on sales. Vicore has appointed Jessica Shull as head of digital therapeutics to lead the VP04 development programme.

Pharmaceutical Technology
JULY 26, 2022
This sales growth will be in line with a steadily increasing disease prevalence and the entrance of novel agents into the market. The three major markets (3MM: the US, Germany and the UK) will increase in market size from $128.35m last year to $188.35m in 2031, at a compound annual growth rate (CAGR) of 3.9%.
Pharmaceutical Technology
MARCH 23, 2023
Seagen specialises in developing antibody-drug conjugates (ADCs) which will complement Pfizer’s oncology portfolio. Seagen will maintain its operations in the Seattle area and will leverage Pfizer’s protein-engineering capabilities to develop next-generation biologics.

XTalks
JANUARY 7, 2025
billion Announced : April 2021 Closed : December 2021 Thermo Fisher Scientific, a worldwide supplier of analytical instruments, clinical development solutions, specialty diagnostics, lab, pharmaceutical and biotech services, acquired PPD , a leading global provider of clinical research services to the biopharma and biotech industry, for $17.4

Pharmaceutical Technology
FEBRUARY 17, 2023
Capricor will handle the clinical development of CAP-1002 and sell commercial products to Nippon Shinyaku. Additionally, Capricor will receive up to $89m in further development and sales-based milestones, along with a double-digit share of revenue based on product sales.

Pharmaceutical Technology
JANUARY 4, 2023
Gilead Sciences has entered a collaboration and licensing agreement with EVOQ Therapeutics to advance the latter’s technology and develop immunotherapies to treat rheumatoid arthritis (RA) and lupus. Under the deal terms, the two companies will work together to advance the preclinical development of RA and lupus immunotherapies.
The Pharma Data
MAY 8, 2021
Sandoz, a Novartis division, today announced progress in the late-stage clinical development program for its proposed biosimilar aflibercept. Sandoz will begin enrolling the first patient in MYLIGHT, a clinical Phase III confirmatory efficacy and safety study, shortly 1. Aflibercept is a key treatment in ophthalmology.

Pharmaceutical Technology
APRIL 25, 2023
Spectrum is a commercial-stage biopharmaceutical company focused on the acquisition, development and commercialisation of novel and targeted oncology treatments. The company has its own clinical development organisation equipped with regulatory and data management competencies.

pharmaphorum
JULY 23, 2021
Syneos Health has appointed Michael Brooks as its new chief development officer. In this newly created role, Brooks will lead the company’s customer engagement and market development portfolio. Brooks has more than 25 years of experience within clinical research and commercialisation.

pharmaphorum
DECEMBER 4, 2020
Pfizer will pay up to $45 million in development related milestone payments and up to $143 million if the vaccine hits early sales targets. Valneva is paying 30% of development costs and in return Pfizer will pay tiered royalties starting at 19% and will lead late-stage development.

Pharmaceutical Technology
JUNE 15, 2023
Verve Therapeutics will be responsible for advancing the research and development of the Lp(a) programme by completing Phase I clinical development. Lilly will handle the subsequent development, manufacture and commercialisation activities of the programme. Lilly will provide funding for the Phase I clinical trials.

pharmaphorum
NOVEMBER 18, 2022
Using CytomX’s Probody and Regeneron’s Veloci-Bi platforms, the collaboration and licensing agreement aims to enable the development of investigational next-generation bispecific immunotherapies. The deal with Regeneron comes months after CytomX Therapeutics laid off 40% of its workers.

Pharmaceutical Technology
FEBRUARY 24, 2023
Applying the drug’s phase transition success rate to remaining R&D costs and likelihood of approval (LoA) to sales related costs provides a risk-adjusted NPV model (rNPV). The rNPV model is a more conservative valuation measure that accounts for the risk of a drug in clinical development failing to progress.

BioTech 365
SEPTEMBER 22, 2020
TPG Capital establishes new entity to continue the development of select clinical assets spun-out of Adare Pharmaceuticals Announcement follows TPG Capital’s sale of Adare Pharmaceuticals’ specialty CDMO pharmaceutical technology and microbiome businesses SAN FRANCISCO & FORT WORTH, Texas–(BUSINESS WIRE)–TPG Capital, … (..)

Pharmaceutical Technology
JUNE 5, 2023
Rezvilutamide is under clinical development by Jiangsu Hengrui Medicine and currently in Phase I for Liver Failure (Hepatic Insufficiency). GlobalData tracks drug-specific phase transition and likelihood of approval scores, in addition to indication benchmarks based off 18 years of historical drug development data.

Pharmaceutical Technology
JUNE 5, 2023
Rezvilutamide is under clinical development by Jiangsu Hengrui Medicine and currently in Phase III for Prostate Cancer. GlobalData tracks drug-specific phase transition and likelihood of approval scores, in addition to indication benchmarks based off 18 years of historical drug development data. Buy the report here.

Pharmaceutical Technology
MARCH 1, 2023
Orvacabtagene autoleucel is under clinical development by Bristol-Myers Squibb and currently in Phase II for Relapsed Multiple Myeloma. GlobalData tracks drug-specific phase transition and likelihood of approval scores, in addition to indication benchmarks based off 18 years of historical drug development data.

Pharmaceutical Technology
FEBRUARY 20, 2023
Applying the drug’s phase transition success rate to remaining R&D costs and likelihood of approval (LoA) to sales related costs provides a risk-adjusted NPV model (rNPV). The rNPV model is a more conservative valuation measure that accounts for the risk of a drug in clinical development failing to progress.

Pharmaceutical Technology
JANUARY 2, 2023
Danicamtiv is under clinical development by Bristol-Myers Squibb and currently in the Phase I and Phase II in clinical pathway. Danicamtiv overview Danicamtiv (MYK-491, DCM-1) is under development for the treatment of dilated cardiomyopathy (DCM), atrial fibrillation and systolic heart failure.

Pharmaceutical Technology
MAY 31, 2023
Setanaxib is under clinical development by Calliditas Therapeutics and currently in Phase III for Primary Biliary Cholangitis (Primary Biliary Cirrhosis). GlobalData tracks drug-specific phase transition and likelihood of approval scores, in addition to indication benchmarks based off 18 years of historical drug development data.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content