Randomization and Trial Supply Management (RTSM) in Clinical Trials: FAQs
Cloudbyz
AUGUST 15, 2024
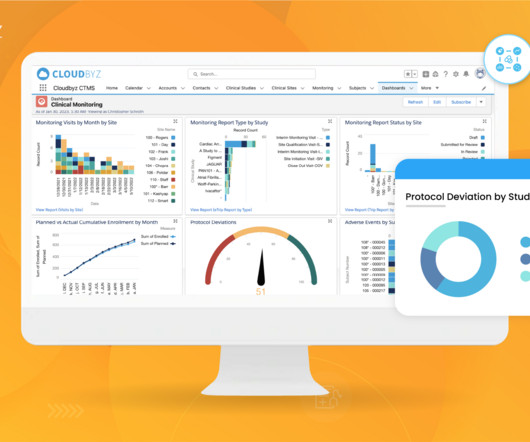
Randomization and Trial Supply Management (RTSM) play a pivotal role in the successful execution of clinical trials. As the complexity of clinical trials continues to increase, especially with the rise of multi-center, adaptive, and decentralized trials, the need for robust RTSM systems has become more critical than ever.











Let's personalize your content