Changing cell & gene therapy landscape and implications on clinical trials
Bio Pharma Dive
APRIL 3, 2023
Cell & gene therapy clinical research may help treat diseases and change how we approach healthcare.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Bio Pharma Dive
APRIL 3, 2023
Cell & gene therapy clinical research may help treat diseases and change how we approach healthcare.
AuroBlog - Aurous Healthcare Clinical Trials blog
SEPTEMBER 9, 2024
The convergence of gene therapies and clinical research is pushing the boundaries of what’s possible in ophthalmic care, offering hope for more effective treatments and potential cures for a range of vision-threatening conditions.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
FEBRUARY 1, 2024
The French government grant will cover preclinical and clinical research for Vivet’s gene therapy for cerebrotendinous xanthomatosis.

Camargo
JULY 30, 2021
Barry Mangum talks the importance of pediatric research, its evolution over the years, and the obstacles and opportunities that the industry faces as it moves forward. How long have you been in pediatric clinical research, and how did you enter the field? So I’ve been working in that space about 43 years.

BioPharma Reporter
DECEMBER 8, 2022
Emmes has announced the creation of a dedicated center for cell and gene therapy research: which will focus on supporting clinical trials for the Clinical Research Organizationâs (CRO) clients.

Pharmaceutical Technology
OCTOBER 26, 2022
The biotherapeutics market is rapidly growing, with 2021 seeing the highest-ever cell and gene therapy approval number. Gene therapy uses DNA to manipulate cells and correct defective genes, whereas cell therapy is the infusion or transplantation of cells into a patient.
Worldwide Clinical Trials
JULY 29, 2024
Partnering with a CRO that emphasizes the complexity of the patient’s voice and harnesses the nuances of clinical trial design is crucial for executing successful clinical research. DEXA (Dual-Energy X-ray Absorptiometry) is the optimal method for accurately measuring body composition in clinical research.

XTalks
MAY 17, 2023
The Foundation for the National Institutes of Health (FNIH) announced this week that the Accelerating Medicines Partnership Bespoke Gene Therapy Consortium (AMP BGTC) has selected eight rare diseases for its clinical trial portfolio.

XTalks
JUNE 8, 2023
Over the past few years, there has been a significant expansion in the cell and gene therapy landscape, with an increasing number of therapies entering clinical trials and receiving regulatory approvals. “I think if we all go into these relationships with this mindset, we’ll achieve great things together.”

AuroBlog - Aurous Healthcare Clinical Trials blog
SEPTEMBER 30, 2024
Researchers see a greater need for new generation therapeutics to propel precision medicine. The development of innovative therapeutic approaches in healthcare encompass a variety of fields, including gene therapy, personalized medicine, immunotherapy, and advanced biologics.

XTalks
JANUARY 16, 2025
Meanwhile, Medtronics BrainSense Adaptive DBS, newly approved in Europe , could help advance neuromodulation therapy for Parkinsons with real-time brain stimulation adjustments as it becomes available in 2025.

Pharmaceutical Technology
JANUARY 17, 2023
The Joint Cancer Immunotherapy Clinical Research Unit of the Hospital Universitario 12 de Octubre and the Spanish National Cancer Research Center (CNIO) created the STAb-T therapy led by Dr Luis Álvarez-Vallina and the Josep Carreras Leukaemia Research Institute team Dr Pablo Menéndez and Dr Diego Sánchez-Martínez.

Scienmag
JANUARY 28, 2021
— In a new study published in Circulation, Mayo Clinic researchers provide the first preclinical, proof-of-concept study for hybrid gene therapy in long QT syndrome, a potentially lethal heart rhythm condition. ROCHESTER, Minn.

Medical Xpress
NOVEMBER 21, 2022
In a new study published in Circulation: Genomics & Precision Medicine, Mayo Clinic researchers designed and developed the first suppression-replacement KCNH2 gene therapy for correcting both long QT syndrome (LQTS) and short QT syndrome (SQTS).

Velocity Clinical Research
OCTOBER 3, 2024
As Nick Spittal states in this Advarra press release, membership in the Gene Therapy Ready (GTR) site network “allows Velocity to start studies over a month faster and provides a meaningful credential and important validation that increases sponsors’ confidence in our specialized capabilities to conduct complex clinical research safely.”
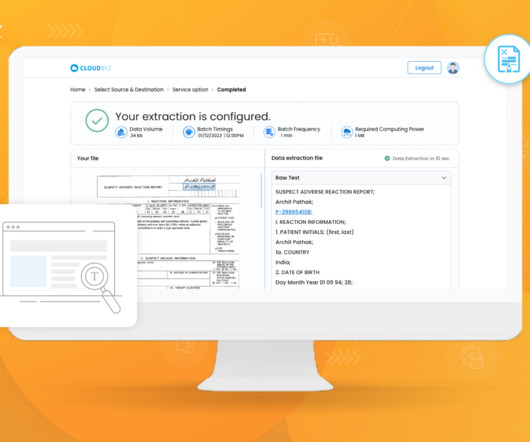
Cloudbyz
JUNE 26, 2023
Generative artificial intelligence (AI) techniques are increasingly being applied in clinical research to enhance various aspects of medical data analysis, diagnosis, treatment planning, and drug discovery. These notes have been used to advance research in NLP and clinical decision support systems.
Worldwide Clinical Trials
NOVEMBER 27, 2023
In recognition of the unmet need and medical urgency for innovative therapies in the sickle cell space, the FDA granted exa-cel Priority Review, with a formal decision expected by December 8, 2023. Why is Casgevy a Breakthrough Gene Therapy for Patients with Sickle Cell Disease?

Advarra
AUGUST 8, 2023
Gene therapy research is booming in the clinical setting. In this blog, we summarize the growth, risks, and regulatory requirements for gene therapy research. We also discuss how a centralized biosafety review process can benefit this type of research.

Outsourcing Pharma
OCTOBER 31, 2022
Thermo Fisherâs clinical research subsidiary, PPD, invests $59 million in sample management and testing capacity for vaccines and cell and gene therapy products with its expansion of laboratory operations in Kentucky.
Pharmaceutical Technology
JUNE 16, 2023
Benefiting from Abu Dhabi’s improved genomics capabilities, the deal seeks to expand research into genomic medicine and genetic diseases to provide patients in the UAE and beyond with improved access to new tools and treatments.

Worldwide Clinical Trials
MAY 20, 2022
The past few years we’ve had exciting opportunities to work on more cell and gene therapy programs for our sponsors. The industry is just at the tip of the iceberg for exploring the potential of these therapies in revolutionizing how we treat disease, and as the approvals start to trickle in, the pipeline only grows.

BioSpace
AUGUST 8, 2021
Voyager’s primary focus will be on developing gene therapies for Huntington’s disease, Amyotrophic Lateral Sclerosis (ALS), and pre-clinical research in spinal muscular atrophy and diseases linked to GBA1 mutations.

Clinical Trial Gurus
AUGUST 26, 2021
Clinical research nurses have the background and the experience to virtually demand a great amount of interest from prospective employers. In this video I interview Michele Blue, a research nurse from an academic medical center (AMC). We discuss her career, job prospects, and a lot of other topics related to clinical research.
Advarra
NOVEMBER 29, 2022
The field of cell and gene therapies (CGT) is constantly evolving, and there has been significant progress in this area of research. However, despite the promise of these therapies, the regulations governing them lag the science, which in turn hinders the clinical translation of these novel medicines.

BioPharma Reporter
FEBRUARY 18, 2021
Massachusetts-based clinical research organization (CRO), Charles River Laboratories, is to acquire Cognate BioServices, a cell and gene therapy contract development and manufacturing organization (CDMO) for around US$875m in cash.

Cloudbyz
JULY 31, 2024
This revolution is enabling the growth of innovative biomarker-based precision medicine and cell and gene therapy, transforming both clinical research and post-market care. These therapies involve modifying a patient’s cells or genes to treat or prevent diseases, with the promise of long-lasting effects.

The Pharma Data
OCTOBER 18, 2020
In late August, Pfizer announced it was investing an additional $500 million into its state-of-the-art gene therapy manufacturing facility in Sanford, North Carolina. Ricci told BioSpace the company has been “investing heavily in gene therapy and in the Raleigh-Durham Research Triangle Park area.”

Advarra
MARCH 20, 2023
The Food and Drug Administration (FDA) recently released new guidance regarding cellular and gene therapy products, one of which may significantly impact early-phase clinical trials of such products. This is to ensure a sufficient level of scientific and clinical understanding is developed prior to Phase I trials.
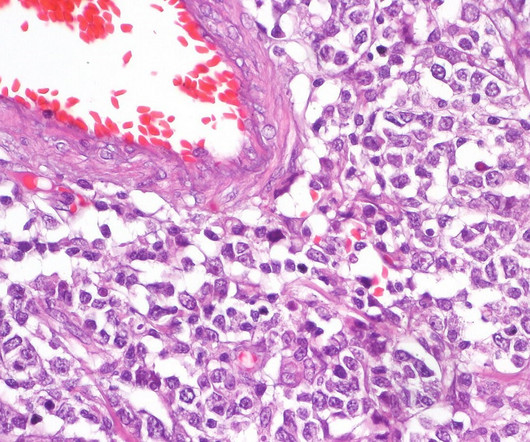
Pharmaceutical Technology
MAY 3, 2023
Both C-CAR039 and the anti-CD20 CAR-T therapy C-CAR066 have been studied in the treatment of non-Hodgkin’s lymphoma and have received investigational new drug (IND) application clearance from the FDA. Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.

Velocity Clinical Research
OCTOBER 4, 2023
Velocity Clinical Research, the leading multi-specialty clinical sites business, announced it is opening greenfield clinical research sites in Bristol, Leicester, and Romford, demonstrating the company’s commitment to the U.K. The sites launch at a time when clinical trial activity in the U.K.

Roots Analysis
AUGUST 29, 2023
Over the years, cell and gene therapies (CGT) and other advanced therapy medicinal products (ATMPs) have managed to capture the interest of both drug developers and healthcare investors. Their popularity can be attributed to various factors, such as minimal side effects, clinical efficacy and their personalized nature.

BioTech 365
DECEMBER 2, 2020
4D Molecular Therapeutics Strengthens Leadership Team with Key Appointments in Clinical Research and Development 4D Molecular Therapeutics Strengthens Leadership Team with Key Appointments in Clinical Research and Development EMERYVILLE, Calif.–(BUSINESS
Cloudbyz
AUGUST 9, 2023
The clinical research landscape is rapidly evolving. As it becomes more complex with growing volumes of data, evolving regulations, and the pressure for faster drug development, traditional methods of clinical research management are no longer sufficient. This is where the platform approach comes into play.
Advarra
JUNE 12, 2023
Gene therapy research is exciting and full of promise, but because of the risks involved, it’s also highly regulated, requiring an institutional biosafety committee (IBC) to provide additional oversight and risk assessment. What Does an IBC Review?
Advarra
MARCH 31, 2023
The use of engineered genetic materials in clinical trials is rapidly expanding, with potential applications for genetic vaccines, gene-modified cellular therapies, and gene therapies. The post Risk Assessment for use of Engineered Genetic Materials in Clinical Research appeared first on Advarra.

Worldwide Clinical Trials
OCTOBER 24, 2023
Seeking relief at the genetic cause of disease Engaged discussion with the audience followed my joint presentation on this topic with Matthew Fuller, PhD, PMP, Executive Director of Gene Therapy Research at Ultragenyx. Roughly 80% of rare diseases are due to a known genetic driver.

XTalks
JANUARY 8, 2024
Whether it’s for a treatment for a chronic ambulatory condition, precision medicine or cell and gene therapy, there is a massive uptick in clinical trial complexity. It’s important to make sure that with this increase in clinical trial complexity, we don’t make our trials overly burdensome to sites or patients,” says Markham.

ACRP blog
FEBRUARY 25, 2025
[ Editors Note: In recognition of Rare Disease Day being observed on February 28, ACRP is pleased to present this, the second of two blogs contributed by subject matter experts offering insights on how rare diseases are being focused on by the clinical research enterprise.

XTalks
DECEMBER 16, 2024
Maria Hopfgarten Head of Global Medical Writing PPD Clinical Research Business of Thermo Fisher Scientific Artificial intelligence (AI) is revolutionizing industries worldwide, and medical writing is no exception. Addressing Risks in AI-Assisted Medical Writing The integration of AI into medical writing isnt without risks.

Velocity Clinical Research
MAY 23, 2024
Standard Velocity site features: Private exam rooms Comfortable patient reception areas Facilities for extended-stay PK studies Ample parking Secure monitoring rooms and workstations equipped with phones and high-speed internet Individual and secure workstations for research staff Regulatory document processing area EDC capabilities Secure record storage (..)

XTalks
FEBRUARY 24, 2023
“Many rare disease communities are seeing gene therapies as potential game changers, since most rare diseases are genetically driven,” Dr. Raymond says. Hence, one conversation that needs to take place is between the sponsors developing gene therapies and the payers who in effect “gate” patient access to these novel treatments.

Advarra
AUGUST 10, 2023
Rapid growth in gene therapy is expected to receive additional support as the Food and Drug Administration (FDA) Center for Biologics Evaluation and Research (CBER) prepares to launch Operation Warp Speed for Rare Diseases. Peter Marks, head of FDA’s CBER – the organization responsible for regulating gene therapies.

XTalks
DECEMBER 21, 2022
The past year has been a strong one for life sciences industries, from pivotal gene therapy approvals to continued innovations in the biotech and medical device spaces. Gene Therapy Approvals. Bluebird bio is a notable mention as it secured FDA approvals for two of its gene therapies, Zynteglo and Skysona.

Cloudbyz
MARCH 22, 2024
In the realm of clinical research, the efficient management of data is paramount. However, traditional methods of data entry, particularly from diverse and unstructured documents, have long posed challenges for researchers, leading to inefficiencies, errors, and compliance risks. Scalability: Cloudbyz’s ClinExtract.AI
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content