Transform Clinical Trial Management with Cloudbyz’s Unified Solution on Salesforce
Cloudbyz
APRIL 30, 2023
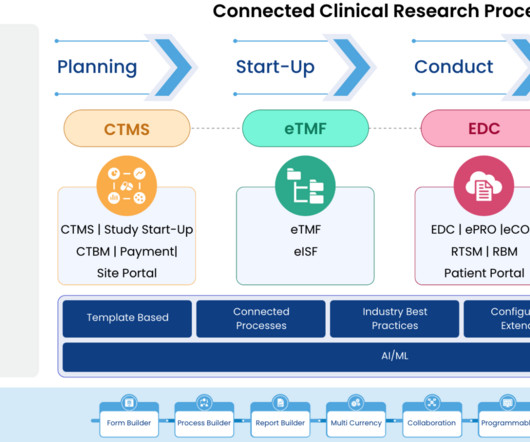
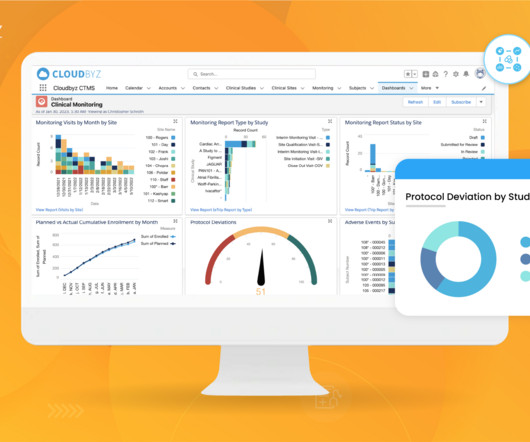
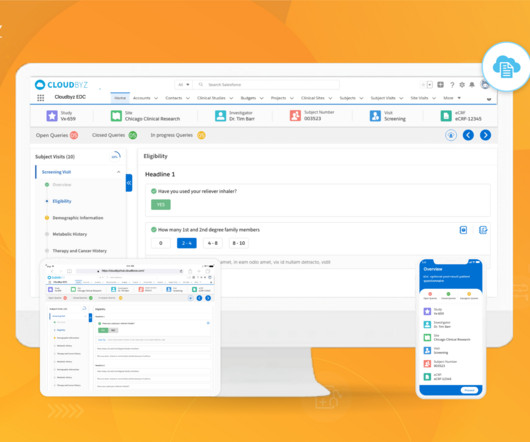
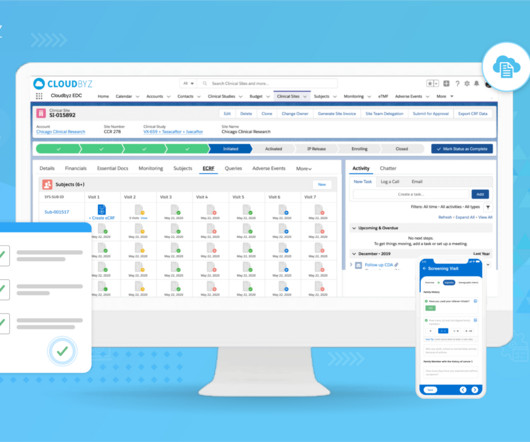
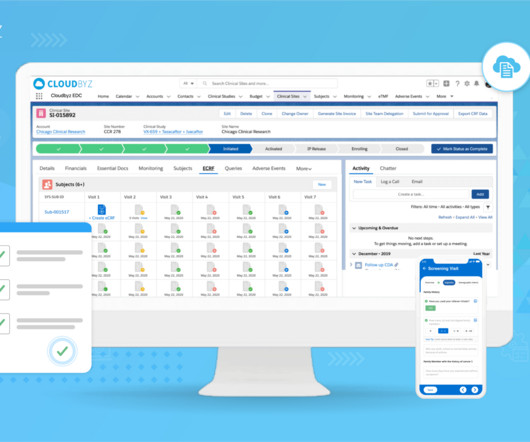
Clinical trials are complex and time-consuming endeavors, requiring efficient management and coordination between various stakeholders. Comprehensive Solution: Cloudbyz’s unified clinical trial management solution covers the entire clinical trial lifecycle, from study planning to close-out.










Let's personalize your content