Gilead adds investigational HIV vaccine to its portfolio from Spanish biotech
Pharmaceutical Technology
NOVEMBER 26, 2024
Gilead and Aelix first teamed up in 2018 for a clinical research collaboration agreement to investigate the vaccine.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
NOVEMBER 26, 2024
Gilead and Aelix first teamed up in 2018 for a clinical research collaboration agreement to investigate the vaccine.
AuroBlog - Aurous Healthcare Clinical Trials blog
AUGUST 3, 2023
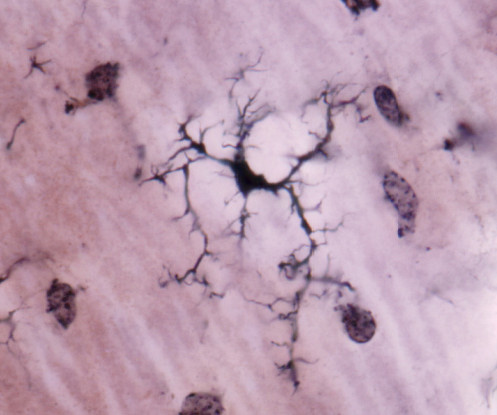
An experimental vaccine that aims to slow down or prevent the progression of Alzheimer’s disease has been trialed in mice with promising early results. Mice engineered with genes that put them at greater risk of an Alzheimer’s-like disease had fewer amyloid plaques following the vaccine.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
AuroBlog - Aurous Healthcare Clinical Trials blog
APRIL 11, 2024
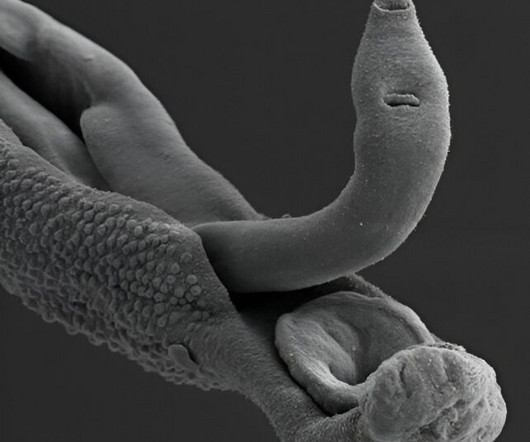
Using viruses that infect bacteria to detect proteins sprouted by a notorious parasite, scientists have honed in on possible vaccine targets for schistosomiasis, a neglected tropical disease that currently affects an estimated 600 million people worldwide, causing 280,000 deaths per year.
AuroBlog - Aurous Healthcare Clinical Trials blog
OCTOBER 8, 2023
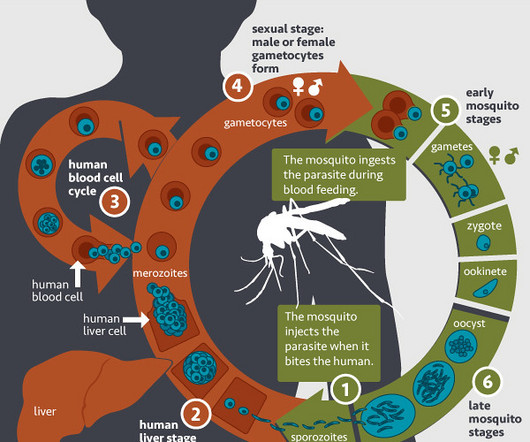
The World Health Organization has approved a new vaccine that scientists argue will be a game-changer in the fight against malaria, which kills half a million people in Africa every year.

AuroBlog - Aurous Healthcare Clinical Trials blog
OCTOBER 16, 2022
The husband-and-wife team who co-founded BioNTech, the biotechnology company that partnered with Pfizer to develop an effective messenger-RNA (mRNA) shot against COVID-19, has predicted that a cancer vaccine could be widely available within the next decade.
AuroBlog - Aurous Healthcare Clinical Trials blog
MARCH 12, 2024
That’s how many vaccines a man in Germany claims to have received for COVID-19 in just 29 months – and his body isn’t reacting the way some scientists thought it would. Two hundred and seventeen. The 62-year-old male from the city of Magdeburg made headlines a few years ago for his private and risky decision […]

AuroBlog - Aurous Healthcare Clinical Trials blog
AUGUST 28, 2023
India’s success story in pediatric vaccination programme should be further replicated in the form of adult vaccination programme, experts recommended on the occasion of the 2nd edition of the India Vaccine Leaders Conclave (IVLC) held between August 22 and August 23, 2023 under the theme “Building Resilient Vaccine Ecosystems” in Mumbai.

AuroBlog - Aurous Healthcare Clinical Trials blog
MAY 14, 2024
A Subject Expert Committee (SEC), which advices the national drug regulator on approvals and clinical trials related to Covid-19 vaccines, has recommended grant of permission to Serum Institute of India (SII) for Omicron XBB 1.5 The vaccine variant […]

AuroBlog - Aurous Healthcare Clinical Trials blog
AUGUST 19, 2024
The Indian Council of Medical Research (ICMR) and Panacea Biotec have announced the initiation of the first-ever phase 3 clinical trial for a dengue vaccine in India. The trial will evaluate the efficacy of India’s indigenous tetravalent dengue vaccine, DengiAll, developed by Panacea Biotec.

AuroBlog - Aurous Healthcare Clinical Trials blog
MAY 13, 2024
The Subject Expert Committee (SEC), which advises the Central drug regulator regarding clinical trials and approvals of drugs, has recommended approval for additional indication of Zydus Lifesciences’ inactivated trivalent influenza vaccine in children above six months with clinical trial waiver subject to condition.

pharmaphorum
FEBRUARY 21, 2022
The clinical research industry has long struggled with participant diversity. One study found that only 5% of Black or Asian United Kingdom residents had ever participated in a clinical trial. A study of FDA-approved vaccine trials from 2011-2020 showed that 78% of participants were white, even though only 60% of the U.S.

AuroBlog - Aurous Healthcare Clinical Trials blog
DECEMBER 14, 2022
The Department of Biotechnology (DBT) is extending support to the institutions and scientists to set up Centres of Excellence in the country for nano-vaccines and nano-adjuvants, to treat cancer and other diseases.The Department has invited Letters of Intent (LoIs) for ‘Setting up of CoE in Nano-Vaccines & Nano-Adjuvants’ for a period of five years (..)

AuroBlog - Aurous Healthcare Clinical Trials blog
JANUARY 22, 2024
The Indian Institute of Science (IISc) is now developing a heat-tolerant vaccine that can offer protection against different strains of SARS-CoV-2 – both current and future variants. The research […]

XTalks
JANUARY 22, 2025
Valneva SE, who developed the worlds first FDA-approved chikungunya vaccine , has now unveiled promising Phase III data for its single-shot chikungunya vaccine, IXCHIQ, in adolescents aged 12 to 17. IXCHIQ is the only licensed chikungunya vaccine in the US for adults aged 18 and older at increased risk of exposure to the virus.

AuroBlog - Aurous Healthcare Clinical Trials blog
OCTOBER 17, 2022
Animal rights organisation People for Ethical Treatment of Animals (PETA) India has urged the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) to withdraw its recent recommendations for using stray dogs for vaccine testing in favour of superior, human-relevant, animal-free research methods, which are more effective (..)

AuroBlog - Aurous Healthcare Clinical Trials blog
JUNE 11, 2023
The Central Drugs Standard Control Organisation (CDSCO) has declared a batch of Typbar, the typhoid polysaccharide vaccine from Bharat Biotech International Ltd as not of standard quality (NSQ).

AuroBlog - Aurous Healthcare Clinical Trials blog
JUNE 25, 2023
The Department of Pharmaceuticals (DoP) has rejected review applications filed by the Maharashtra-based Bharat Serums and Vaccines Ltd against National Pharmaceutical Pricing Authority (NPPA) fixing the ceiling price of its infertility treatment drug brand Hucog HP, since the company did not file the petition within the prescribed time limit.

pharmaphorum
MARCH 1, 2022
The primary care setting offers huge potential for commercial research. This was, and still is being, demonstrated through the UK’s COVID-19 vaccine research successes. Primary care sites require their own approach, but they can have great benefits for clinical trial recruitment, access to hard-to-reach patients, and more.

pharmaphorum
NOVEMBER 15, 2022
A renaissance in vaccines research is just one example of something good to come out of the pandemic. For the clinical trials industry, the pandemic forced new ways of working and fresh ways of thinking about clinical research and how to conduct it remotely.

Velocity Clinical Research
FEBRUARY 11, 2025
Velocity is a nominee for two 2025 Vaccine Industry Excellence (ViE) Awards! After winning the Best Clinical Trial Company ViE Award in 2024, we would be honored to receive your vote and once again share this recognition with you as we work to improve vaccine trials worldwide.

AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 13, 2023
COVID sent us into a series of lockdowns in a bid to control the spread of the virus until a vaccine could be developed. Most of us knew that a vaccine would be the key to our post-pandemic […] The world changed for all of us when we were suddenly plunged into a pandemic in 2020.

pharmaphorum
JANUARY 21, 2022
A third dose of vaccine has been shown to effectively neutralise the Omicron variant of covid-19, according to researchers. Research led by UCLH, UCL and the Francis Crick Institute found that the booster dose successfully stimulates antibody levels that neutralise Omicron. Overall, antibody levels were nearly 2.5

Velocity Clinical Research
MARCH 19, 2025
Charles Eger, MD, contributed to new research on a combination vaccine intended to provide protection against both seasonal flu and COVID-19 in a single shot. Full article: [link] The post Charles Eger, MD, Authors New Article in Nature Medicine appeared first on Velocity Clinical Research.

XTalks
OCTOBER 18, 2022
A clinical research organization (CRO) is a company that delivers outsourced services to plan, manage and execute clinical trials for biotechnology, pharmaceutical, and medical device companies. Read on to learn why life science and clinical research professionals are choosing a career with Medpace. Cardiovascular.
AuroBlog - Aurous Healthcare Clinical Trials blog
JANUARY 21, 2024
With a fatality rate 20 times that of COVID-19, and no vaccine, Disease X could swiftly bring humanity to its knees. Few would be left untouched by the pathogen were it to gain hold, causing healthcare systems to crumble and economies to collapse as the world once again tried to contain a force of nature. […]

AuroBlog - Aurous Healthcare Clinical Trials blog
NOVEMBER 24, 2022
The WHO said the aim was to update a list used to guide global research and development (R&D) and investment, especially in vaccines, tests, and […].

DM Clinical Research
SEPTEMBER 29, 2020
Annual flu shots are very important, as are other vaccines. While vaccines are quick and easy, they sometimes might require a little down-time. If you are dealing with a headache, fever, or nausea after your vaccine, then it is best to skip the gym. Are Vaccine Side Effects Normal? Vaccines Matter- Now More than Ever.
pharmaphorum
JULY 1, 2021
Miruna Sasu lays out how real-world data is evolving in the post COVID-19 vaccine reality. . Only a year after the emergence of COVID-19, a handful of life science companies started publishing data on the encouragingly high efficacy of their vaccine candidates. The speed with which these vaccines came to the public is remarkable.

XTalks
SEPTEMBER 5, 2023
Vaccines have been an integral piece of the global public health toolbox for over 200 years, but the COVID-19 pandemic brought about a new era in vaccine development with renewed interest in mRNA technology and unprecedented accelerated regulatory approvals. What are the major challenges you’re facing right now in vaccine development?

Outsourcing Pharma
OCTOBER 31, 2022
Thermo Fisherâs clinical research subsidiary, PPD, invests $59 million in sample management and testing capacity for vaccines and cell and gene therapy products with its expansion of laboratory operations in Kentucky.
XTalks
AUGUST 13, 2020
As the rush for a COVID-19 vaccine continues, healthcare services company Kaiser Permanente has joined in on the clinical testing of Pfizer Inc. and BioNTech’s lead vaccine candidate in a Phase III trial being conducted at sites in California and Oregon.

WCG Clinical
OCTOBER 8, 2024
Latin America’s genetic diversity and unique health challenges are driving groundbreaking clinical research, offering new insights into disease prevention, innovative medicine, and treatments. 4] Cultural Exchange and Medicine Vaccines are an example of how cultural exchange and collaboration shape global health advancements.

Medical Xpress
DECEMBER 1, 2022
In 2021, a group of scientists led by researchers at the University of North Carolina at Chapel Hill, Weill Cornell Medicine and NewYork-Presbyterian reported that the Moderna mRNA vaccine and a protein-based vaccine candidate containing an adjuvant, a substance that enhances immune responses, elicited durable neutralizing antibody responses to SARS-CoV-2 (..)

ACRP blog
OCTOBER 10, 2023
In recognition of October being Breast Cancer Awareness Month , ACRP recently went behind the scenes with a clinical research coordinator on the study team for a bold Cleveland Clinic investigation of a vaccine aimed at preventing triple-negative breast cancer (TNBC), the most aggressive and lethal form of the disease.

Pharma Mirror
JANUARY 23, 2023
This webinar focused on the need for a coordinated clinical trials enterprise, one that can quickly characterize emerging viral threats and evaluate the effectiveness of vaccines, therapeutics, and other countermeasures across a diversity of trial participants.

AuroBlog - Aurous Healthcare Clinical Trials blog
SEPTEMBER 22, 2022
The Indian Institute of Science (IISc), Centre for BioSystems Science and Engineering, has designed a new method to deliver a vaccine candidate for tuberculosis (TB). The research involves using spherical vesicles secreted by bacteria coated on gold nanoparticles which can then be delivered to the immune cells.

Velocity Clinical Research
NOVEMBER 12, 2024
Aiming to revitalize the country’s pharmaceutical industry, it signaled a shift from policymakers on clinical research and drug development issues. The strategy outlined several measures to address the country’s declining role in global clinical trials, safeguard pharmaceutical supply chains, speed up approvals, and reduce bureaucracy.

AuroBlog - Aurous Healthcare Clinical Trials blog
FEBRUARY 12, 2023
The Central government has notified the administrative Rules for the Committee for Control and Supervision of Experiments on Animals (CCSEA), the statutory Committee to regulate and monitor experiments on animals to prevent cruelty to animals, with provisions for deemed approval and grading of animal house facilities in medical colleges, pharmacy colleges, (..)

AuroBlog - Aurous Healthcare Clinical Trials blog
SEPTEMBER 28, 2022
The Delhi High Court has quashed four demand notices raised by the National Pharmaceutical Pricing Authority (NPPA) against Bharat Serums and Vaccines Ltd and medical devices manufacturer Bard Healthcare India Pvt Ltd for overcharging on non-scheduled drugs and medical devices, finding the move as unjustifiable as it does not allow rounding off principle (..)

STAT News
FEBRUARY 17, 2023
Pfizer and partner Valneva are scrapping data from roughly half of the participants in a Phase 3 trial of a Lyme disease vaccine candidate after uncovering quality issues with a third-party clinical trial operator.

Velocity Clinical Research
MAY 21, 2024
“Next to clean water, vaccines are probably responsible for more saved lives than anything else we’ve done in medicine.” ” -Mark Turner, MD, Vaccine CARE Council Leader and Velocity Principal Investigator in Boise, ID.

Pharma Times
MARCH 15, 2024
More than 3,700 cases of mpox have been identified in the UK since May 2022
pharmaphorum
JANUARY 5, 2022
A COVID-19 vaccine that could work against multiple variants of the coronavirus – developed by US biotech Gritstone bio – has generated encouraging immune response data in its first clinical trial. “But it may be; and if it doesn’t other novel vaccines with similar benefits may well take on this role.”

Velocity Clinical Research
APRIL 24, 2024
He’s also a leading expert for the Vaccine CARE Council. But as he explains in this video, he’s excited about the opportunities the CARE Council model is creating for investigators, even beyond vaccines. Shishir Khetan , MD, is a Principal Investigator and Senior Medical Director at Velocity.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content