Altasciences' comprehensive, integrated solution for clinical supply manufacture
Bio Pharma Dive
DECEMBER 7, 2020
Integrated CRO/CDMO solutions for accelerated and more efficient early drug development programs.

Bio Pharma Dive
DECEMBER 7, 2020
Integrated CRO/CDMO solutions for accelerated and more efficient early drug development programs.
Pharmaceutical Technology
MAY 24, 2023
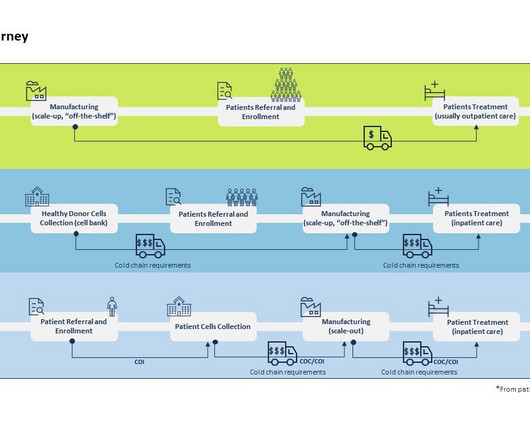
Every major pharma company is now involved in CGT development which has resulted in the approval of 28 therapies by the FDA thereby making CGT no longer a niche category of therapies. Allogeneic therapies begin with healthy donor samples to develop the eventual therapeutic product which can be administered to multiple patients.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
MAY 15, 2023
NUCLIDIUM has signed a strategic collaboration deal with PharmaLogic for the manufacturing and clinical supply of copper-based theranostics ⁶¹Cu in the US. ⁶¹Cu The deal is set to speed up the development of NUCLIDIUM’s theranostic pipeline. The deal is set to speed up the development of NUCLIDIUM’s theranostic pipeline.

The Pharma Data
MAY 26, 2023
The expansion forms part of Catalent’s ongoing global strategy to increase its ability to handle, store and manage advanced therapies for clinical supply, and follows investments at its facilities in Philadelphia, Singapore, and Shanghai, China, in specialized, ultra-low temperature storage capabilities. With sites in the U.S.,
Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial.
Worldwide Clinical Trials
NOVEMBER 12, 2024
Despite their exciting potential, the smooth operation of cell therapy development trials requires extraordinary orchestration, perfectly aligning the product and patient journeys. While clinical supply is essential to any successful trial, autologous cell therapy trials occupy the far end of the spectrum regarding risk tolerance.

Outsourcing Pharma
AUGUST 15, 2022
Signant Health has partnered with the software company o connect its platforms for the management of clinical supply chain inventory, labels, and content.
Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials.
Let's personalize your content