Untangling the Complexities of Cell and Gene Therapy Clinical Trials: A Supply Chain Perspective
Pharmaceutical Technology
MAY 24, 2023
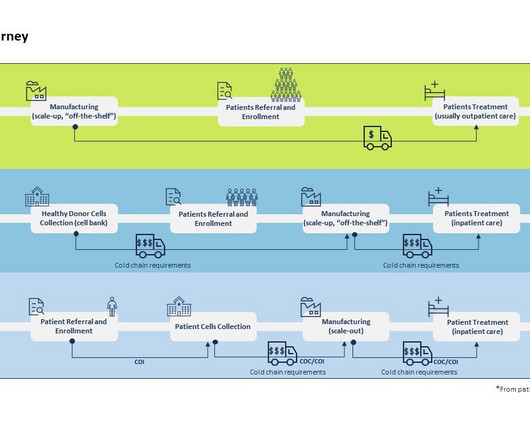
By Luisa Sterkel & Joana Loureiro , Tenthpin Consultants The promise and potential of cell and gene therapies (CGT) has emerged in the recent past and currently over 1.500 CGT are registered for clinical trials holding great hope for the treatment of challenging and uncurable diseases.










Let's personalize your content