Comprehensive Guide to Medical Device Safety, Systems, and Regulatory Compliance: FAQ
Cloudbyz
AUGUST 19, 2024
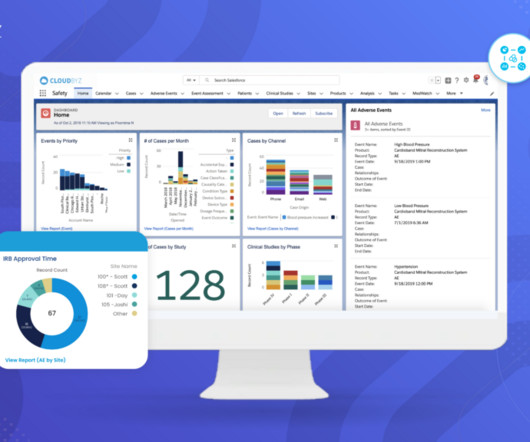
Manufacturers must adhere to a wide array of standards and guidelines that dictate how devices are developed, tested, and brought to market. Compliance with their regulations is mandatory for manufacturers seeking to market their devices globally. What is the role of post-market surveillance in medical device safety?









Let's personalize your content