ICON and LEO Pharma join forces on clinical trial mission
Pharma Times
MARCH 10, 2023
Collaboration will concentrate on launching and running studies within medical dermatology
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharma Times
MARCH 10, 2023
Collaboration will concentrate on launching and running studies within medical dermatology

Pharmaceutical Technology
APRIL 13, 2023
Following the increased use of telemedicine during the Covid-19 pandemic, the potential of digital technologies in communication, data collection, and analysis has become increasingly realised by patients, healthcare systems, and clinical trial sponsors.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Rethinking Clinical Trials
JUNE 26, 2024
Mirza is chief resident in the Department of Dermatology at the Warren Alpert Medical School of Brown University. The post June 26, 2024: Using ChatGPT to Facilitate Informed Medical Consent, in This Week’s PCT Grand Rounds appeared first on Rethinking Clinical Trials. Join the online meeting.
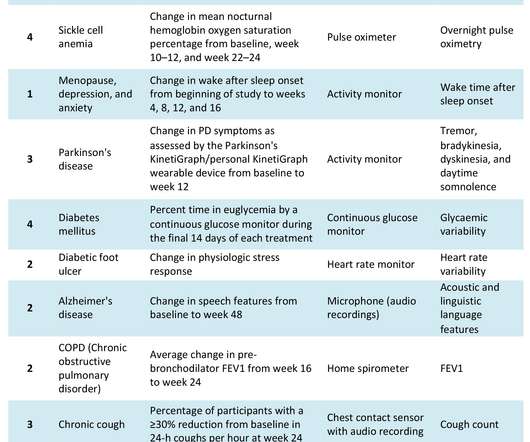
pharmaphorum
JULY 8, 2022
The COVID-19 pandemic has catalysed significant changes in the way pharma develops drugs, particularly in the clinical trial space. Hybrid or decentralised clinical trials (DCTs) have gained traction as technology, infrastructure and knowledge have evolved to support their use. Source: Izmailova et al, 2017.

FDA Law Blog
NOVEMBER 10, 2024
Each year, FDA selects a limited number of clinical trials to fund to help sponsors pursue development of medical products for rare diseases and advance their field. In October, FDA announced seven new clinical trial grants awarded in fiscal year (FY) 2024 – including one for a Phase 3 trial – totaling $17.2

Velocity Clinical Research
JULY 18, 2023
Born and trained in Egypt, he moved to the UK several years ago after working as a general practitioner there and deciding he loved the country and the clinical trial opportunities it offered. To join a clinical trial at Velocity, visit velocityclinicaltrials.com.

Pharmaceutical Technology
MAY 19, 2023
Clinical response was observed after just two weeks in some cases. The FDA nod means Rinvoq is now approved for seven indications in gastroenterology, rheumatology and dermatology. A maintenance study showed the same effects at 52 weeks. In patients with severe symptoms, a dosage of 30mg can be considered.
STAT News
DECEMBER 2, 2022
And a fragmented clinical trials industry has made it a prime target for private equity, which often consolidates markets by merging companies.

VirTrial
JULY 7, 2024
In recent years, there has been significant activity in the AD clinical trial landscape, including the approval of novel systemic therapies. Accurate measurement of AD severity over time is crucial for evaluating treatment efficacy in these trials. JAMA Dermatology , 149 (5), 577-582. References: Armstrong, A.

Pharmaceutical Technology
JULY 12, 2022
A validated target in dermatology, PDE4 is an enzyme that induces overactive immune responses. The NDS submission by Arcutis is based on positive findings from the company’s pivotal Phase III programme and two long-term open-label clinical trials. Health Canada has provided a target action date of 30 April next year.

VirTrial
JULY 7, 2024
In recent years, there has been significant activity in the AD clinical trial landscape, including the approval of novel systemic therapies. Accurate measurement of AD severity over time is crucial for evaluating treatment efficacy in these trials. JAMA Dermatology , 149 (5), 577-582. References: Armstrong, A.

VirTrial
JULY 7, 2024
In recent years, there has been significant activity in the AD clinical trial landscape, including the approval of novel systemic therapies. Accurate measurement of AD severity over time is crucial for evaluating treatment efficacy in these trials. JAMA Dermatology , 149 (5), 577-582. References: Armstrong, A.

Outsourcing Pharma
FEBRUARY 2, 2021
While the technology has been found mostly in dermatological applications, its use in studies is expanding, according to Illingworth Research Group.

WCG Clinical
OCTOBER 9, 2023
The dermatology market is embarking on tremendous growth and sponsors need to be ready. Over the last 12 months, dermatology-focused studies have increased by roughly 20%, with top focus areas including psoriasis, atopic dermatitis, and alopecia. This rapid growth brings new challenges. Efficiency and speed are critical.

Pharmaceutical Technology
FEBRUARY 28, 2023
Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. GlobalData tracks drug-specific phase transition and likelihood of approval scores, in addition to indication benchmarks based off 18 years of historical drug development data.

Delveinsight
SEPTEMBER 28, 2021
Innovent, UNION Therapeutics Set to Advance Dermatology Market in China. Innovent Biologics and UNION therapeutics have announced a strategic and licensing collaboration to develop and commercialize Orismilast , a next-generation PDE4 inhibitor for inflammatory dermatology conditions in China.

pharmaphorum
JULY 5, 2022
Trial organisers face intense competition to find and recruit eligible patients for atopic dermatitis studies. With more than 500 dermatology clinical trials currently underway, it is often heard that there is a ‘’competition for participants’’. Patient recruitment for moderate or severe atopic dermatitis.
Olympian Clinical Research
FEBRUARY 26, 2021
Clinical trials are research studies designed to evaluate or test a medical, surgical, or behavioral intervention. Most recently, clinical trials have gained recognition through the development and testing of Covid-19 vaccines. Today we’re taking some time to highlight a few of the biggest ones!
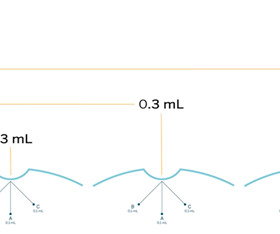
WCG Clinical
MARCH 30, 2023
Uniform Live Action Training Using InvestigatorSpace Speeds Up Study Start Times In one case, a global pharmaceutical company used WCG’s training solutions to support two dermatology studies they were running. Study teams engaged with the training content throughout the trial and asked questions through the InvestigatorSpace platform.

XTalks
SEPTEMBER 30, 2024
Presented at the European Academy of Dermatology and Venereology (EADV) Congress 2024, the study highlighted significant improvements in key disease markers, positioning orismilast as a potential breakthrough in atopic dermatitis management.

VirTrial
JULY 7, 2024
In recent years, there has been significant activity in the AD clinical trial landscape, including the approval of novel systemic therapies. Accurate measurement of AD severity over time is crucial for evaluating treatment efficacy in these trials. JAMA Dermatology , 149 (5), 577-582. References: Armstrong, A.

WCG Clinical
SEPTEMBER 20, 2023
Welcome to the recorded session of ‘Optimizing Endpoint Assessments for Dermatology Studies.’ In this insightful webinar, we take a deep dive into dermatology trials and discuss how standardizing rater qualification and training can solve common challenges and protect the quality and integrity of endpoint data.

XTalks
APRIL 17, 2023
In clinical trials, patients experienced significantly clearer skin and less interference with sleep due to itch when taking lebrikizumab compared to placebo. The ADvocate1 and ADvocate2 Phase III studies were published in the New England Journal of Medicine and the British Journal of Dermatology , respectively.

Rethinking Clinical Trials
SEPTEMBER 24, 2024
Mirza, MD, MPH Chief Resident Department of Dermatology Warren Alpert Medical School of Brown University Slides Keywords Artificial Intelligence; ChatGPT; Informed Consent Key Points Artificial Intelligence (AI), when implemented thoughtfully in clinical settings, can lead to meaningful results for patients.

Pharmaceutical Commerce
JANUARY 16, 2024
Zoryve (roflumilast) shows efficacy in seborrheic dermatitis and atopic dermatitis in clinical trials.

XTalks
MARCH 18, 2025
Incytes recent announcement on the topline results from two Phase III clinical trials of povorcitinib in patients with hidradenitis suppurativa has stirred both hope and caution in the market.
XTalks
JUNE 20, 2022
The results of their Phase IIa STP705 clinical study were published in the Journal of Drugs in Dermatology , a peer-reviewed medical journal of dermatology that was established in 2002. Clinical Trial Outcomes of isSCC Treatment with STP705. STP705 — A Promising siRNA Candidate Against isSCC.
XTalks
AUGUST 2, 2023
Eichenfield was also the principal investigator on clinical trials evaluating YCANTH. Verrica Pharmaceuticals is a dermatology therapeutics company developing medications for skin diseases that require medical interventions.

Scienmag
MAY 21, 2021
The findings of a clinical trial by Trinity College Dublin researchers of treatment for atopic dermatitis have been published today in The Lancet journal (Friday, 21st May, 2021). Results of the clinical trial at […].
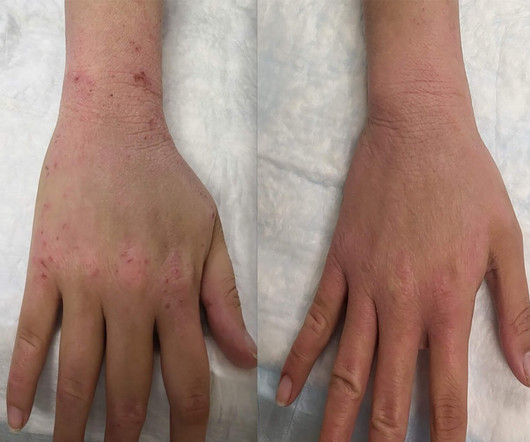
Medical Xpress
MARCH 14, 2025
Scientists from NIH's National Institute of Allergy and Infectious Diseases (NIAID) identified treatments that could be studied in clinical trials for the condition based on their potential to lower levels of the chemical compoundcalled nicotinamide adenine dinucleotide (NAD+), a form of vitamin B3.

XTalks
JUNE 26, 2024
Hyperhidrosis is the third most prevalent dermatological condition in the US, affecting roughly 15.3 While Sofdra stands out as the first new chemical entity approved for primary axillary hyperhidrosis, the market for hyperhidrosis treatments includes other medications and clinical trials.

ECRG Media's Clinical Research Podcast
SEPTEMBER 11, 2018
A german CRO that specializes in Dermatology clinical trials has decided to open an office in North Carolina. Not in the Biotech and clinical research hub of the Raleigh/Durham Triangle but in the coast near Wilmington. Have you heard about ProInnovera before or have an interest in Dermatology?

ECRG Media's Clinical Research Podcast
SEPTEMBER 11, 2018
A german CRO that specializes in Dermatology clinical trials has decided to open an office in North Carolina. Not in the Biotech and clinical research hub of the Raleigh/Durham Triangle but in the coast near Wilmington. Have you heard about ProInnovera before or have an interest in Dermatology?

Pharmaceutical Technology
FEBRUARY 28, 2023
Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. GlobalData tracks drug-specific phase transition and likelihood of approval scores, in addition to indication benchmarks based off 18 years of historical drug development data.

The Pharma Data
OCTOBER 29, 2020
30, 2020 /PRNewswire/ — Eli Lilly and Company (NYSE: LLY) and Incyte (NASDAQ: INCY) announced today new data for baricitinib (marketed as OLUMIANT ® ) will be presented at the annual Fall Clinical Dermatology meeting taking place virtually October 29-November 1, 2020. 10x ULN were observed in patients in Olumiant clinical trials.

XTalks
AUGUST 2, 2024
These trials reveal nemolizumab’s potential to significantly improve key aspects of atopic dermatitis, including skin lesions, itch and sleep disturbance, in both adolescent and adult patients with moderate-to-severe cases.

XTalks
MARCH 24, 2023
The company recently presented a pooled analysis from clinical studies of their DermaSensor skin cancer detection device at the American Academy of Dermatology (AAD) 2023 Annual Meeting. The results presented were from two of DermaSensor’s four clinical studies that were submitted to the US Food and Drug Administration (FDA).
XTalks
MAY 31, 2022
XTALKS WEBINAR: All Means All: The Road to Inclusivity in Clinical Trials. Register for this free webinar to learn what the FDA’s new draft guidance means for diverse and inclusive trials. Clinical Trials of Vtama. At Dermavant, we are committed to advancing novel, patient-focused innovation in immuno-dermatology.

Pharma Marketing Network
APRIL 30, 2021
Clinical trials may be one of the very last bastions of a non-customer-focused approach within the pharmaceutical industry. Across the board, from rheumatology to neurology, outcomes that matter most to patients, have traditionally not been captured in clinical trials. How do we increase the diversity of clinical trials?

Pharmaceutical Technology
FEBRUARY 28, 2023
Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. GlobalData tracks drug-specific phase transition and likelihood of approval scores, in addition to indication benchmarks based off 18 years of historical drug development data.

Pharmaceutical Technology
FEBRUARY 28, 2023
Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. GlobalData tracks drug-specific phase transition and likelihood of approval scores, in addition to indication benchmarks based off 18 years of historical drug development data.

Pharmaceutical Technology
FEBRUARY 24, 2023
Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. GlobalData tracks drug-specific phase transition and likelihood of approval scores, in addition to indication benchmarks based off 18 years of historical drug development data.

Pharmaceutical Technology
FEBRUARY 24, 2023
Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. GlobalData tracks drug-specific phase transition and likelihood of approval scores, in addition to indication benchmarks based off 18 years of historical drug development data.
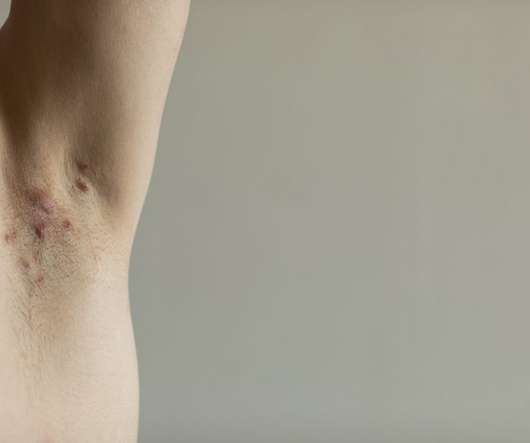
Olympian Clinical Research
AUGUST 5, 2020
Although severe cases of HS can appear unsightly, with intense bruising or discoloration, pus, and boils; the condition is merely a type of dermatological condition the same as acne, psoriasis, and countless other skin conditions. . HS is not caused by lack of hygiene.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content