Avrobio receives orphan drug designation for Hunter syndrome gene therapy
Pharmaceutical Technology
JULY 14, 2022
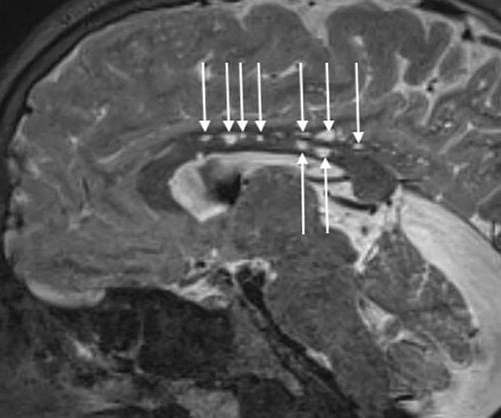
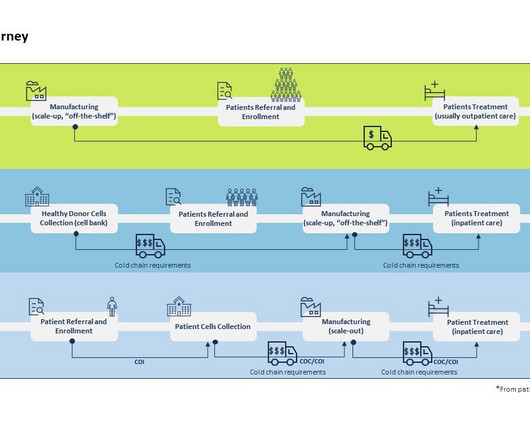
Avrobio has received orphan drug designation for its gene therapy, AVR-RD-05, from the US Food and Drug Administration (FDA) to treat mucopolysaccharidosis type II (MPSII) or Hunter syndrome. The company noted that this gene therapy is the fourth one to receive orphan drug designation.






































Let's personalize your content