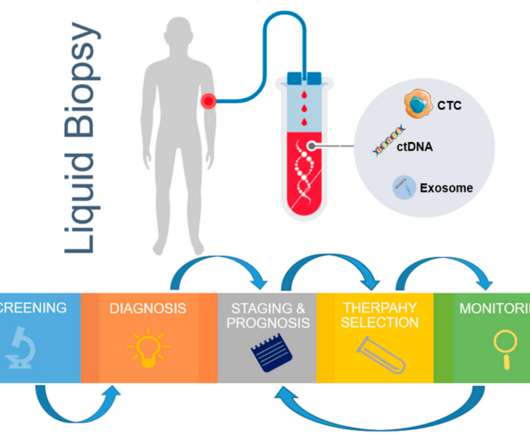
The Utility of Liquid Biopsy in Oncology Clinical Trials
XTalks
NOVEMBER 7, 2022
Liquid biopsies have demonstrated promising activity in multiple clinical settings, but the great enthusiasm and expectations around them can be harmful if not adequately managed,” said Dr. El Mustapha Bahassi, PhD, Director, Clinical Laboratory Sciences, at the global clinical research organization (CRO) Medpace.























Let's personalize your content