FDA approves Alzheimer’s drug from Eisai, Biogen in closely watched decision
Bio Pharma Dive
JANUARY 6, 2023
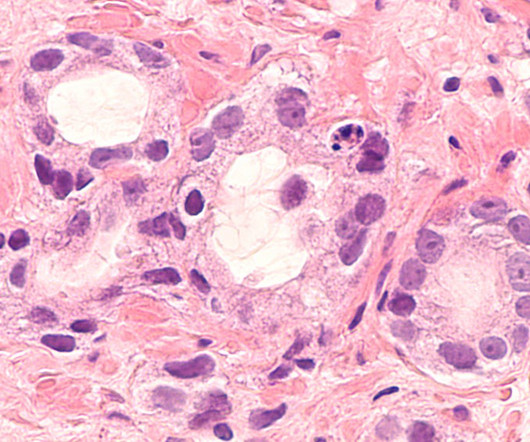
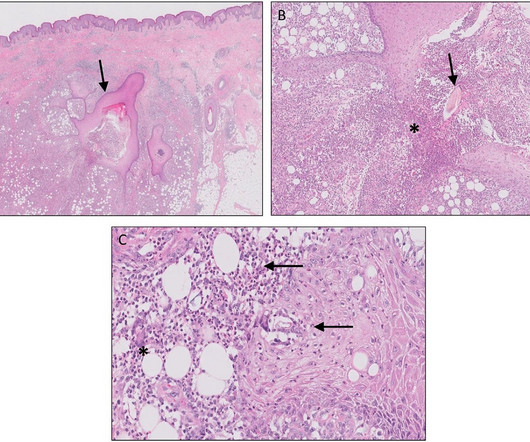
The agency’s approval comes months after a large clinical trial showed the drug, called Leqembi, could slow the disease’s progression. Yet experts have raised concerns about its safety.





































Let's personalize your content