Jesduvroq (daprodustat) is FDA Approved for Anemia due to Chronic Kidney Disease
XTalks
FEBRUARY 6, 2023
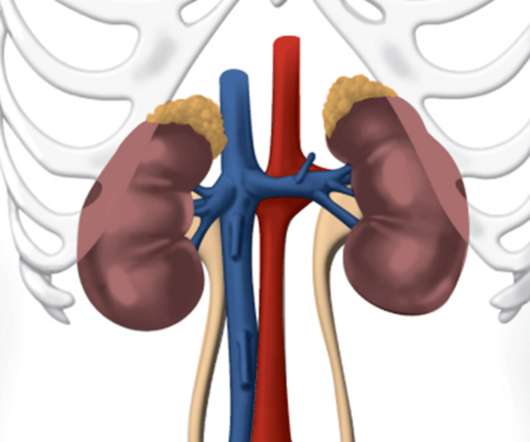
GlaxoSmithKline LLC (GSK) recently announced that the US Food and Drug Administration (FDA) approved Jesduvroq (daprodustat), a new once-a-day oral treatment for anemia due to chronic kidney disease (CKD). The approval is currently only for adult patients who have been undergoing dialysis for at least four months.




























Let's personalize your content