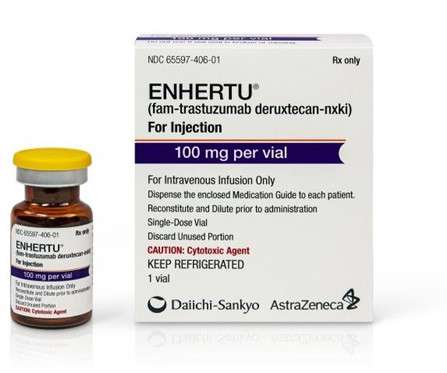
AstraZeneca-Daiichi Sankyo’s Enhertu gets US FDA approval for breast cancer
Pharmaceutical Technology
AUGUST 8, 2022
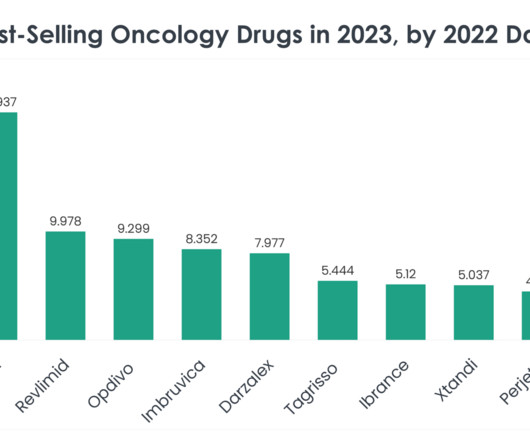
The approval was based on findings from the international, randomised, open-label Phase III DESTINY-Breast04 clinical trial. Furthermore, Enhertu’s safety profile was in line with prior clinical trials without any new safety concerns detected. months compared with 5.1 months for the chemotherapy arm.


































Let's personalize your content