8 key clinical trials to watch for the rest of 2021
Bio Pharma Dive
JUNE 21, 2021
The next six months could feature clinical milestones for CRISPR gene editing, the treatment of COVID-19, microbiome drugs and gene-targeted cancer therapy.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Bio Pharma Dive
JUNE 21, 2021
The next six months could feature clinical milestones for CRISPR gene editing, the treatment of COVID-19, microbiome drugs and gene-targeted cancer therapy.
Worldwide Clinical Trials
NOVEMBER 27, 2023
Casgevy, the commercial product formerly known as exa-cel, is administered by taking stem cells out of a patient’s bone marrow and editing a gene in the cells in a laboratory, with the modified cells then infused back into the patient after conditioning treatment to prepare the bone marrow.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Rethinking Clinical Trials
JULY 31, 2024
Adrian Hernandez In this Friday’s PCT Grand Rounds, Adrian Hernandez of Duke University will present “Precision Health to Population Health: Opportunities and Challenges for Gene Editing Therapies.” He is a co–principal investigator of the NIH Pragmatic Trials Collaboratory Coordinating Center.

pharmaphorum
JANUARY 29, 2021
Cutting edge’ is, for once, a truly apt description when it comes to gene editing – both because the field is pushing medicine into areas we might never have dreamed possible, and because these technologies involve literally cutting DNA at a specific point in the genome. Zinc fingers. That would just slow the whole field down.
Worldwide Clinical Trials
NOVEMBER 12, 2024
For example, once considered incurable and terminal, patients with sickle cell disease may reach new summits in their lives with gene editing technologies such as CRISPR to repair affected DNA and, in some cases, functionally cure the condition. Cell therapy, specifically, is changing our narrative around chronic and lethal conditions.
BioPharma Reporter
NOVEMBER 16, 2021
have received the green light from Health Canada for their Clinical Trial Application for VCTX210: an allogeneic, gene-edited, immune-evasive, stem cell-derived therapy for the treatment of type 1 diabetes (T1D). CRISPR Therapeutics and ViaCyte, Inc.

Pharmaceutical Technology
JUNE 15, 2023
Verve Therapeutics and Eli Lilly and Company have entered an exclusive research partnership to advance the former’s preclinical stage in vivo gene editing programme targeting lipoprotein(a) (Lp(a)) to treat atherosclerotic cardiovascular disease (ASCVD). Lilly will provide funding for the Phase I clinical trials.

STAT News
NOVEMBER 7, 2022
Verve Therapeutics said Monday that its experimental gene-editing treatment for a common form of heart disease was placed on clinical hold by the Food and Drug Administration, potentially delaying an ongoing, early-stage clinical trial. The Cambridge, Mass.-based Continue to STAT+ to read the full story…

AuroBlog - Aurous Healthcare Clinical Trials blog
SEPTEMBER 17, 2024
We are witnessing a revolution in healthcare, driven by advances in genetics, Omics, RNA and CRISPR gene-editing technology, to deliver precision and personalised medicine, said Kiran Mazumdar-Shaw, executive chairperson, Biocon and Biocon Biologics. Biology is opening up new frontiers in medicine.

BioSpace
APRIL 29, 2024
The FDA has cleared a clinical trial of an ex vivo prime editing candidate in patients with a rare disease, Prime Medicine announced Monday. The technique taps CRISPR technology to rewrite defective genes without breaking DNA double helix strands.

pharmaphorum
FEBRUARY 1, 2024
A single dose of a gene-editing drug being developed by Intellia Therapeutics almost eliminated attacks in patients with hereditary angioedema (HAE) in a clinical trial, pointing to the potential of CRISPR-based drugs delivered in vivo to treat diseases.

pharmaphorum
SEPTEMBER 28, 2022
Vertex Pharma and partner CRISPR Therapeutics will start a rolling marketing application in the US for their gene-editing drug for sickle cell disease (SCD) and beta thalassaemia later this year. The time places exa-cel in pole position to become the first drug developed based on CRISPR/Cas9 gene-editing technology to reach the market.

pharmaphorum
OCTOBER 8, 2020
Scribe Therapeutics, a start-up focusing on gene-editing using CRISPR/Cas9, has burst onto the biotech scene with a $415 million deal with Biogen. Further back in development are drugs that will be administered to edit genes within the body, but the first of these candidates are now in clinical trials.
pharmaphorum
JUNE 12, 2022
A gene-editing drug developed by CRISPR Therapeutics and Vertex Pharma has continued to show impressive results in clinical trial, with an update at the EHA congress showing long-term effects on the symptoms of both beta thalassaemia and sickle cell disease. billion product if it gets approved for both indications.
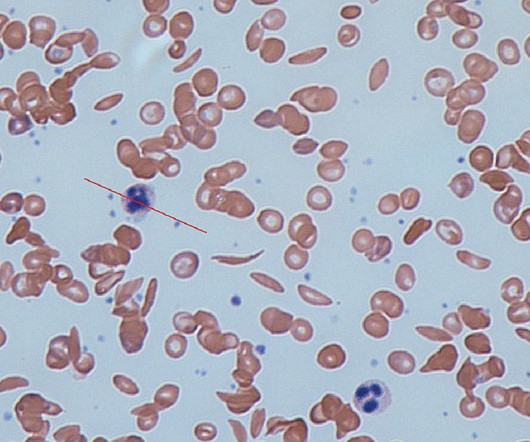
Pharmaceutical Technology
APRIL 4, 2023
Formerly known as CTX001, exa-cel is an investigational, autologous, ex vivo CRISPR/Cas9 gene-edited therapy. The BLAs are supported by data obtained from the ongoing Phase III CLIMB-111 and CLIMB-121 trials, along with an ongoing long-term follow-up CLIMB-131 trial.

Pharmaceutical Technology
AUGUST 24, 2022
A lead product candidate of the company, GTP-506 is a single-dose gene editing therapy. The ARCUS nuclease vector (GTP-506A) targets gene editing in the PCSK9 gene locus that is well characterised, while the therapeutic donor vector (GTP-506D) introduces the OTC gene to provide the required genetic correction.

Camargo
JULY 27, 2021
Gene therapy is a new therapeutic approach in which genes are used to treat or prevent diseases. It is a comprehensive term which encompasses a large variety of therapy products including viral and bacterial vectors, plasmid DNA, human gene editing technology, and patient-specific cellular gene therapy.

STAT News
NOVEMBER 4, 2022
A man with muscular dystrophy who was first in line to receive an experimental gene editing therapy tailor made to treat the cause of his rare form of the disease has died. Terry had long been too old to participate in clinical trials of experimental therapies for the disease, which are often geared towards young boys.

Scienmag
JANUARY 19, 2021
publishers New Rochelle, NY, January 19, 2021–Gene editing therapies, including CRISPR-Cas systems, offer the potential to correct mutations causing inherited retinal degenerations, a leading cause of blindness. Credit: Mary Ann Liebert, Inc.,

Worldwide Clinical Trials
OCTOBER 24, 2023
Global Genes is a rare disease umbrella organization dedicated to eliminating the burdens and challenges of rare diseases for patients and families globally. The Therapeutic Strategy Leads for Rare Disease at Worldwide Clinical Trials have been working alongside and championing these organizations for many years.

pharmaphorum
JUNE 23, 2022
Novartis has shouldered its way into the in vivo gene editing category via a deal with US biotech Precision BioSciences, focused on a therapy for sickle cell disease (SCD). It is also working with Eli Lilly on in vivo gene-editing drugs against three targets, including one in Duchenne muscular dystrophy, under the terms of a $1.4
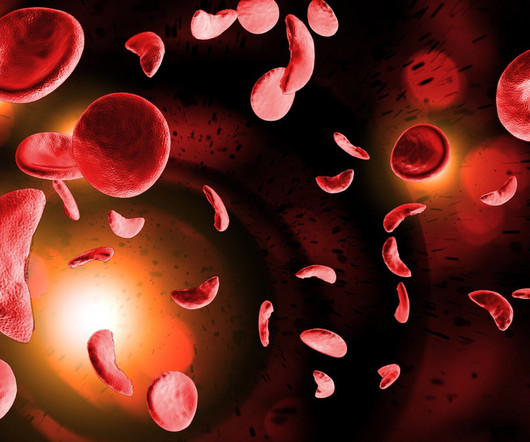
Pharmaceutical Technology
APRIL 28, 2023
EDIT-301 is made of patient-derived CD34+ haematopoietic stem and progenitor cells, which are edited using CRISPR at the gamma globin gene (HBG1 and HBG2) promoter sites using a proprietary engineered AsCas12a nuclease, per the company’s website. This increases the expression of fetal haemoglobin.

Worldwide Clinical Trials
MAY 30, 2024
Authors: Rich Worldwide Clinical Trials Exec. This trend is on the rise despite recent disappointments with clinical trial outcomes, which have the potential to destabilize the industry in the short term regarding drug development strategy and optimal study designs. Director, Therapeutic Area Medical Lead.

XTalks
AUGUST 29, 2022
Now a common gene editing tool, the popularity of the CRISPR-Cas9 system has increased over the past decade. CRISPR is notable for engineering living cells, allowing scientists to edit, turn off, delete, or replace genes in a cell’s genome.

BioSpace
OCTOBER 14, 2021
Read about TFF Pharmaceuticals and Augmenta Bioworks' dry powder COVID-19 antibody formula, the world's first gene editing clinical trial for PKU, Bayer's COVID-19 vaccine and other key developments in life sciences research.
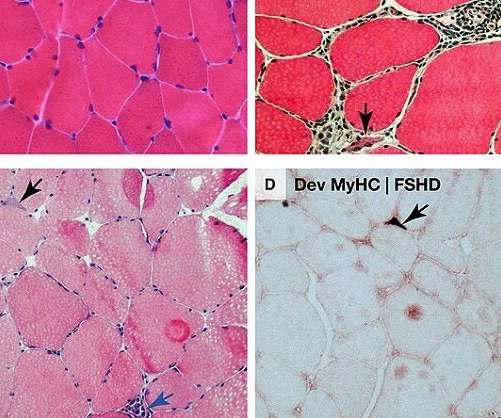
Pharmaceutical Technology
OCTOBER 13, 2022
Vita Therapeutics intends to use the funding to advance its lead pre-clinical programme VTA-100 for limb-girdle muscular dystrophy (LGMD2A) to clinical trials. Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva. The company has raised nearly $66m in total since its inception.

pharmaphorum
OCTOBER 7, 2020
Drs Emmanuelle Charpentier and Jennifer Doudna have won this year’s Nobel Prize for chemistry in recognition of their work on the gene-editing technology CRISPR/Cas9. million) Nobel Prize award. “In

Pharmaceutical Technology
MARCH 2, 2023
Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. The company develops gene-editing organ transplantation technology. QN-023a overview QN-023a is under development for the treatment of relapsed or refractory acute myeloid leukemia (AML).

Pharmaceutical Technology
MARCH 2, 2023
Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. The company develops gene-editing organ transplantation technology. Hangzhou Qihan Biotechnology overview Hangzhou Qihan Biotechnology is a healthcare company.

WCG Clinical
OCTOBER 21, 2024
Clinical trials are the cornerstone of advancing medical research, but behind the science, the experiences of participants and their families offer invaluable insight into how research impacts real lives. Advances in research, such as gene editing and cellular therapies, have opened new treatment possibilities for both conditions.
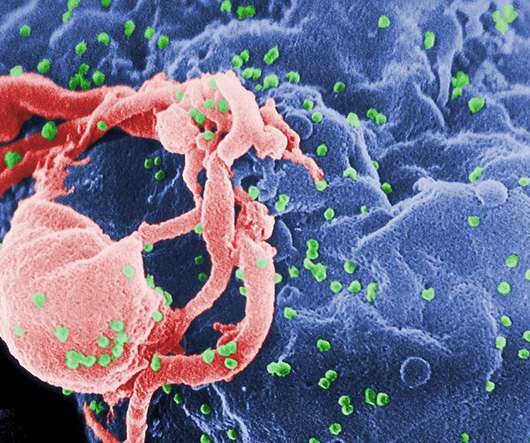
pharmaphorum
FEBRUARY 19, 2021
Snipping out this viral code with powerful CRISPR gene editing technology, which last year won Drs Emmanuelle Charpentier and Jennifer Doudna the Nobel Prize for chemistry, would in theory prevent the need for drugs to suppress the virus and the development of AIDS.
pharmaphorum
FEBRUARY 3, 2021
Dr Jennifer Harbottle, senior scientist in the R&D Base Editing team of PerkinElmer’s Horizon Discovery business, looks at progress made in the realms of biotechnology and next-generation diagnostics, vaccines and therapeutics, including the application of CRISPR-Cas9 gene editing in developing and refining cell therapies.
pharmaphorum
SEPTEMBER 29, 2020
As summarised by The National Institutes of Health, this includes reducing the number of redundant clinical trials, enhancing the statistical strength of studies, reducing overall costs and risks, and improving study participant recruitment, all while triggering creativity and innovation between collaborators.

Pharmaceutical Technology
MARCH 1, 2023
Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. Merck also provides a wide range of products including lab water systems, gene editing tools, cell lines, antibodies and end-to-end systems.

Pharmaceutical Technology
MARCH 1, 2023
Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. Merck also provides a wide range of products including lab water systems, gene editing tools, cell lines, antibodies and end-to-end systems.
XTalks
FEBRUARY 10, 2023
According to a recent review published in Nature , as of April 2022 almost 1,800 active cell therapy clinical trials were listed in ClinicalTrials.gov, a 33 percent increase from 2021. In a recent webinar, Dr. Vassallo discussed the operational considerations for complex cell therapy clinical trials.

XTalks
DECEMBER 20, 2023
This includes analyzing how drug combinations may impact individual patients or groups of patients before even entering a clinical trial. With a staggering 90 percent of drugs failing in clinical trials, AI has the potential to help improve these statistics.

Pharmaceutical Technology
JANUARY 19, 2023
While allogeneic hematopoietic stem cell transplants (HSCT) provide long-term benefit to a subset of AML patients, other cell therapy modalities such as natural killer (NK) cells or chimeric antigen receptor (CAR)-T cells have failed to show conclusive benefit in late-stage clinical trials.
XTalks
JANUARY 26, 2024
Innovations in Cancer Therapy CRISPR/Cas9, a groundbreaking gene-editing technology, has demonstrated significant potential in oncology, offering new avenues for cancer treatment. Clinical trials have demonstrated the potential of CRISPR/Cas9 in this field.

pharmaphorum
NOVEMBER 23, 2021
It is also close to Cambridge University, seen as one of the world’s leading life sciences research centres, as well as the city’s Addenbrookes Hospital, an important centre for clinical trials in the National Health Service.

pharmaphorum
JULY 13, 2021
Novo Nordisk also gets rights to the remainder of Dublin-based Prothena’s ATTR amyloidosis programme under the terms of the deal, although PRX004 is the only candidate that has reached the clinical trials stage. All told, the deal could be worth up to $1.2

pharmaphorum
MARCH 19, 2021
Around $7 to $9 million from the proceeds of the IPO will be used to advance Gain’s preclinical-stage candidates for Morquio and GM1 into phase 1/2 clinical trials, according to the prospectus for the offering. The post Protein misfolding specialist Gain nets $40m from IPO appeared first on.

XTalks
OCTOBER 20, 2023
Clinical-stage genome editing company Intellia Therapeutics has received clearance from the US Food and Drug Administration (FDA) for its Investigational New Drug (IND) application to start a pivotal phase III trial of NTLA-2001 for the treatment of transthyretin (ATTR) amyloidosis with cardiomyopathy.

pharmaphorum
AUGUST 8, 2022
CRISPR Therapeutics and Vertex are also in the running with their gene-editing candidate CTX001, in phase 1/2 trials which are due to generate final results later this year. GBT’s inclacumab – another P-selectin antibody that could encroach on Adakveo – is in a pair of phase 3 trials due to generate results next year.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content