Oramed hits oral insulin clinical trial milestone
BioPharma Reporter
MAY 5, 2022
Oramed Pharmaceuticals announced this week that it has enrolled 100% of the patients in the worldâs first Phase 3 study of oral insulin under FDA approved protocols.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
BioPharma Reporter
MAY 5, 2022
Oramed Pharmaceuticals announced this week that it has enrolled 100% of the patients in the worldâs first Phase 3 study of oral insulin under FDA approved protocols.

XTalks
APRIL 15, 2025
In the pancreas, potassium channels regulate insulin secretion. Vykay XR Clinical Trial Results Data from a randomized, placebo-controlled Phase III trial (Study 2-RWP or Study C602-RWP) support Vykat XRs approval. Based on the average weight of patients in its clinical trials, Vykat XR will cost $466,200 per year.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

XTalks
DECEMBER 13, 2024
Petrelintide, administered once a week under the skin, targets obesity through its action as an amylin analog, a hormone co-secreted with insulin from the pancreas. Amylin helps regulate satiety and slows gastric emptying, which can support weight loss.

pharmaphorum
JULY 7, 2021
Three months ago, the FDA said it was unconvinced by a bridging study designed to show equivalence between Provention’s product intended for commercial sale, made by contract manufacturer AGC Biologics, and the drug that teplizumab’s original developer Eli Lilly used in clinical trials.
pharmaphorum
JUNE 18, 2021
Teasing out complex relationships within data lies at the heart of any digital health intervention, but finding the algorithms to do so reliably – and making sure they will be acceptable to regulators – can be a challenge. The algorithms used in healthcare are becoming more complex as well.

FDA Law Blog
JULY 10, 2024
Lenz, Principal Medical Device Regulation Expert — FDA’s Center for Devices and Radiological Health (CDRH) recently partnered with the Digital Medicine Society (DiMe) to host a two-day workshop to help advance the use of patient-generated health data (PGHD) to support improved clinical trials, medical device development, and regulatory science.

pharmaphorum
MAY 21, 2021
Arecor’s own lead product is an ultra-rapid acting insulin, which the company hopes will outperform market rivals from the likes of Eli Lilly, Sanofi and Novo Nordisk. Codenamed AT247, the product has a novel meal-time insulin formulation, which significantly accelerates insulin absorption post injection.
XTalks
JULY 15, 2024
The drug has been evaluated in five clinical trials for PBH. Congenital HI is a genetic disorder that causes excessive insulin production by the pancreas, which lowers plasma or blood sugar. This action helps mitigate hypoglycemia by reducing insulin secretion and stabilizing glucose levels.
XTalks
JULY 15, 2024
The drug has been evaluated in five clinical trials for PBH. Congenital HI is a genetic disorder that causes excessive insulin production by the pancreas, which lowers plasma or blood sugar. This action helps mitigate hypoglycemia by reducing insulin secretion and stabilizing glucose levels.

pharmaphorum
JUNE 14, 2021
Rebecca Sanders from Lipodystrophy UK tells us how the patient voice helped convince NICE to approve a much-needed drug for this rare disease, and explores how regulators and pharma companies can help make patient involvement in HTA more impactful. This article appears in our free digital magazine Deep Dive: Market Access 2021.

XTalks
APRIL 29, 2022
Eli Lilly announced that its obesity drug tirzepatide has scored favorably in a late-stage clinical trial, with results showing that people who took the drug lost an average of 50 pounds, or 21 percent, of their body weight compared to placebo.
pharmaphorum
MARCH 21, 2022
Now, the regulator has accepted the resubmission, kicking off another six-month review period as teplizumab has previously been awarded breakthrough status. Teplizumab is thought to work by binding to CD3, preventing the activation of T cells that attack and kill insulin-producing pancreatic beta cells in the autoimmune disease.

XTalks
FEBRUARY 6, 2024
Revita, an outpatient endoscopic procedural therapy, which aims to eliminate insulin needs and enhance glycemic control by ablating dysfunctional duodenal mucosa. Fractyl Health has initiated discussions with European regulators to establish an investigational new drug (IND)-enabling pathway for RJVA-001 in the treatment of T2D.

The Pharma Data
MARCH 3, 2022
Patients taking the oral blood pressure medication not only required less daily insulin two years after first diagnosis of the disease, but also showed evidence of surprising immunomodulatory benefits. Type 1 diabetes is an autoimmune disease that causes loss of pancreatic beta cells, which produce endogenous insulin.
pharmaphorum
NOVEMBER 18, 2022
The US regulator has approved the anti-CD3 antibody as Tzield to delay the onset of stage 3 T1D in people eight years and older who currently have stage 2 disease, which according to the FDA “may provide patients with months to years without the burdens of disease.”

The Pharma Data
DECEMBER 15, 2020
As the world’s first PPAR pan agonist that completed two confirmatory phase III clinical trials, Chiglitazar Sodium has shown significant and long-lasting hypoglycemic effects in a series of clinical studies, as well as other comprehensive effects including significant insulin sensitization and blood lipid regulation.
XTalks
JANUARY 21, 2025
The tricuspid valve, one of the heart’s four valves, regulates blood flow from the right atrium to the right ventricle, preventing backflow between these chambers. In a clinical trial, participants demonstrated a 98% success rate after six months post implantation, with arteries widened successfully and no stent fractures observed.

XTalks
SEPTEMBER 17, 2020
In rare disease trials, it’s not always feasible to choose clinically-relevant endpoints to measure the efficacy of a new therapeutic. Metabolic changes associated with lipodystrophy include insulin resistance, glucose intolerance, diabetes and hypertriglyceridemia.
XTalks
JANUARY 25, 2023
Brenzavvy’s approval was based on an impressive amount of data collected from 23 clinical trials involving over 5,000 patients with type 2 diabetes. A drug intended for use in animals is called a new animal drug and the FDA’s Center for Veterinary Medicine (CVM) approves and regulates new animal drugs.

The Pharma Data
JANUARY 19, 2021
The vast majority have type 2 diabetes — which arises when the body loses its sensitivity to the blood-sugar-regulating hormone insulin. 13 in the journal BMJ , pooled the results of 23 clinical trials involving more than 1,300 people that tested low-carb diets against other options — often a low-fat diet.
Delveinsight
AUGUST 20, 2020
regulator that had earlier told there was no requirement of an AdComm for the drug, aka, valrox, has issued a complete response (CRL) letter, which could cause a significant delay for any future approval, and canceled what would have been the first-ever approval for gene therapy in the bleeding disorder.

The Pharma Data
DECEMBER 15, 2020
The researchers regulated the abnormal immunological memory processes found in these patients. Treatment induced sustainable clinical responses and reduced systemic inflammation. The human monoclonal antibody targets specific immune plasma cells. Daratumumab already is approved for the treatment of multiple myeloma.
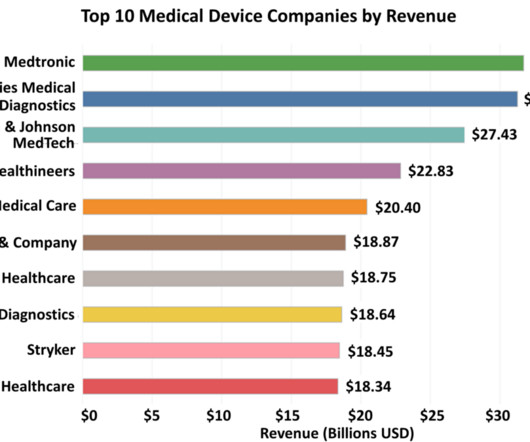
XTalks
JUNE 12, 2023
Medtronic’s proactive stance towards R&D is evident from the over 230 clinical trials it conducted and the more than 200 regulatory approvals it received in the US, Europe, Japan and China in the 2022 fiscal year. Internationally, Medtronic expanded its MiniMed 780G insulin pump and Guardian 4 sensor.

The Pharma Data
DECEMBER 13, 2020
The Company additionally announced new preclinical results and plans for a Phase 2b trial focused on patients with noncirrhotic biopsy-proven NASH and coexisting prediabetes or T2DM. In the T2DM subpopulation, PXL770 was generally safe and well tolerated and was similar to the whole trial population. .

XTalks
FEBRUARY 8, 2021
While there are currently six biosimilars for AbbVie’s Humira (adalimumab) that have been approved by the regulator, the company’s patents prevent biosimilars from being launched until 2023. The regulator allows biosimilars to show slight differences in clinically inactive components of a product.

Scienmag
DECEMBER 3, 2020
Researchers from the University of Copenhagen and biotech firm Gubra have developed a new insulin molecule that will make blood sugar regulation both easier and safer for those with type 1 diabetes Everyday life for the more than 46 million people around the world who suffer from type 1 diabetes could become much easier and […].

XTalks
NOVEMBER 9, 2022
GLP-1 receptor agonists, or incretin (metabolic hormones) mimetics, are analogs of the GLP-1 peptide hormone that binds to the GLP-1 receptor to regulate blood sugar levels by boosting insulin secretion. dependent insulin secretion and suppression of glucagon release. What Are GLP-1 Agonists? mediated glucose?dependent
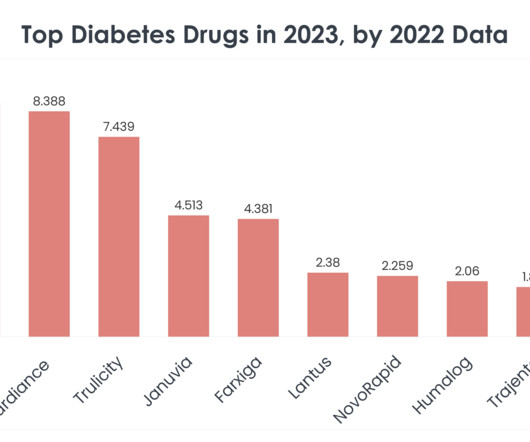
XTalks
FEBRUARY 8, 2024
In its 2022 financial report, Sanofi reported that sales of the long-acting insulin dropped 27.6 percent in the US, impacted by prior formulary losses as well as “erosion of the basal insulin market,” the company said in the statement. In its 2022 financial report, Sanofi reported that sales of the long-acting insulin dropped 27.6

Druggist
MAY 6, 2021
Beta-cells : cells present in the pancreas, which produce insulin. Insulin: a hormone that allows glucose (sugar) to be used for energy in the body. Lastly, metformin improves sensitivity to insulin (Wiernsperger & Bailey, 1999), which regulates body sugar levels. Metformin does not increase insulin release.

pharmaphorum
DECEMBER 14, 2020
Armed with new data from a randomised clinical trial, one company is hoping to change that mindset. The company is FriendsLearn – based in India and with an R&D office in San Francisco – which recently reported results from a trial showing that its fooya!

XTalks
JUNE 10, 2024
Efruxifermin is a novel Fc-FGF21 fusion protein designed to emulate the activity of native FGF21, an endogenous hormone that regulates metabolism and alleviates cellular stress. Numerous compounds are currently under clinical investigation for MASH. This comprehensive action of efruxifermin addresses the multifaceted nature of MASH.

The Pharma Data
JUNE 8, 2023
”No matter the individual, all had increased taurine levels after exercise, which suggests that some of the health benefits of exercise may come from an increase in taurine,” Yadav says.Only a randomized clinical trial in people will determine if taurine truly has health benefits, Yadav adds. ” Source link: [link]

The Pharma Data
AUGUST 3, 2021
The company announced positive top-line results from the SURPASS-4 Phase 3 clinical trial of tirzepatide in adults with type 2 diabetes, evaluating A1C and body weight reductions from baseline. The trial compared tirzepatide to insulin glargine in adults with type 2 diabetes and increased cardiovascular risk. 23% 13,545.7

The Pharma Data
DECEMBER 15, 2020
The Phase III trial evaluated the use of the diabetes vaccine Diamyd ® , an antigen-specific immunotherapy based on the auto-antigen GAD (glutamic acid decarboxylase), to induce immunological tolerance and stop the autoimmune destruction of insulin producing cells. People with type 1 diabetes cannot produce insulin.

Delveinsight
AUGUST 25, 2021
They are governed by a number of rules and regulations that govern drug patenting, testing, safety, efficacy, and marketing. . There are 23 therapies and vaccines in total that are in Phase II of clinical trials. Pharmaceutical companies may deal in both generic and brand-name drugs as well as medical devices. trillion in 2021.
The Pharma Data
MAY 13, 2021
Insulin is a hormone that’s produced in your pancreas…. And you’ve almost certainly heard of insulin before…. You don’t need to be a diabetic to have insulin challenges…. Because as world renowned health expert Dr. Mark Hyman puts it: “No contest: The monster hormone that causes weight gain is excess insulin.”.

XTalks
AUGUST 2, 2023
In September 2022, Roche acquired Good Therapeutics for an upfront payment of $250 million, and has access to their PD-1-regulated IL-2 program. It is a dipeptidyl peptidase-4 inhibitor that keeps insulin levels stable and reduces the amount of glucose produced by the body. Merck’s total global revenue from Lagevrio was $5.68

The Pharma Data
MAY 13, 2021
Insulin Resistance and Cancer Risk: An Overview of the Pathogenetic Mechanisms. Ectopic lipid storage and insulin resistance: a harmful relationship. Relationship between disease severity, hyperinsulinemia, and impaired insulin clearance in patients with nonalcoholic steatohepatitis. Reprod Toxicol. 2014 Nov;49:196-201.

XTalks
MAY 20, 2022
The injection is intended to regulate blood sugar, in conjunction with diet and exercise, in adults with type 2 diabetes. Mounjaro’s FDA approval was based on data from five clinical trials either as a single therapy or in combination with other diabetes medications. percent more than insulin degludec and 1.0
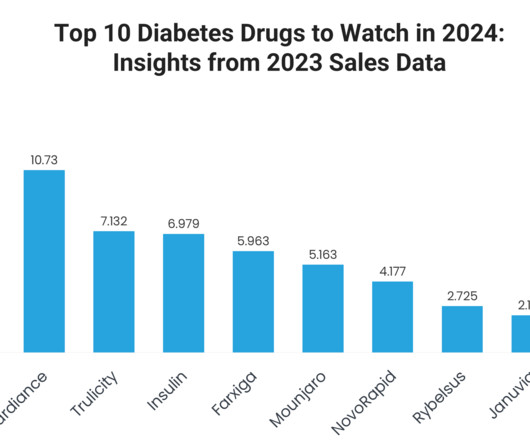
XTalks
DECEMBER 3, 2024
In this blog, we take a closer look at the top 10 diabetes medications based on recent sales trends, exploring the factors behind their success, key clinical benefits and their broader impact on patient care. Price of Insulin: Varies significantly by type and brand of insulin. The company is targeting revenue of $40.4

The Pharma Data
MAY 17, 2021
Clinical trial strategies for rare neurodevelopmental disorders: challenges and opportunities ( Nature ). Viatris Expects First “Interchangeable” Designation in July 2021 For Insulin Products ( Big Molecule Watch ). EU Medical Device Regulation May Spur Litigation Uptick ( Law360 ).
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content