Type 1 Diabetes Vaccine: Does Diamyd Have a Winning Formula?
XTalks
SEPTEMBER 15, 2020
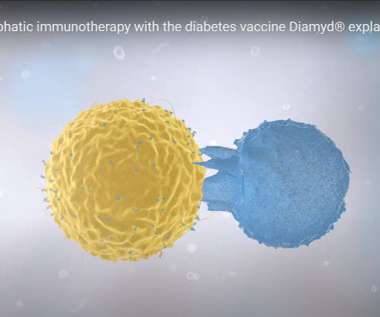
A vaccine developed by Swedish company Diamyd Medical has demonstrated significant treatment efficacy in a predefined genetic subgroup of individuals with type 1 diabetes in a Phase IIb clinical trial. Diamyd Medical is a clinical-stage biopharmaceutical company that develops therapies for type 1 diabetes.
























Let's personalize your content