Pharma companies must adapt to keep pace with AI developments, say experts
Pharmaceutical Technology
OCTOBER 25, 2024
At the Outsourcing in Clinical Trials Conference, key opinion leaders shared predictions for workflow changes due to AI.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
OCTOBER 25, 2024
At the Outsourcing in Clinical Trials Conference, key opinion leaders shared predictions for workflow changes due to AI.

Bio Pharma Dive
OCTOBER 1, 2024
Medical meetings often feature important clinical trial results, making them barometers of biotech and pharma companies' research progress. Here’s a list of conferences to watch in 2025.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
MAY 24, 2023
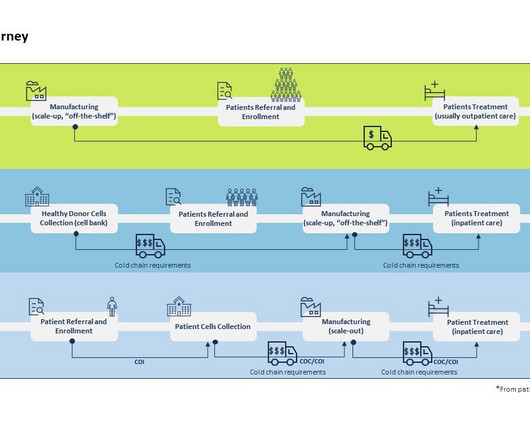
By Luisa Sterkel & Joana Loureiro , Tenthpin Consultants The promise and potential of cell and gene therapies (CGT) has emerged in the recent past and currently over 1.500 CGT are registered for clinical trials holding great hope for the treatment of challenging and uncurable diseases.

AuroBlog - Aurous Healthcare Clinical Trials blog
AUGUST 3, 2023
The Institute is now looking for partners in pharma companies to pursue further clinical development of the findings. When the compound was tested […]

Pharma Times
MARCH 24, 2021
Report outlines two cases for using synthetic data in the clinical trial process

AuroBlog - Aurous Healthcare Clinical Trials blog
MARCH 29, 2023
Indian healthcare providers and pharma companies pace up their efforts with early detection and research for new medicines to combat the spread of tuberculosis as drug resistance concerns emerge. TB is a significant public health threat, with an estimated 10 million annual cases. India shares the highest TB burden with 2.69

Pharmaceutical Technology
JUNE 15, 2023
But before pharmaceutical companies can go to market with a breakthrough drug, they need to ensure safety and efficacy through clinical trials. Pharma R&D teams are solving this problem by leveraging the power of artificial intelligence (AI) in clinical trials to save time and money.

Pharmaceutical Technology
MARCH 6, 2023
Incannex Healthcare has collaborated with New Jersey-based pharma company Catalent for the development and manufacturing of a cGMP-grade psilocybin drug product for clinical trials and potential commercial use. It will also establish cGMP manufacturing of a drug product which will be utilised in future clinical trials.

pharmaphorum
AUGUST 24, 2022
Diversity in clinical trials is a “scientific imperative”, but how can industry bridge the gap between “why” and “how”? Removing barriers to clinical trial participation for underserved groups is an essential part of addressing health inequalities. Clinical trial participation, she went on, was vital.

Pharmaceutical Technology
JANUARY 16, 2023
Moreover, Insilico Medicine’s INS018_055, an AI-designed candidate, has produced positive topline results in its Phase I clinical trial for idiopathic pulmonary fibrosis (IPF) treatment. In addition to collaborating with AI-technology providers, pharma companies are also engaging in vertical integration.
pharmaphorum
JULY 8, 2022
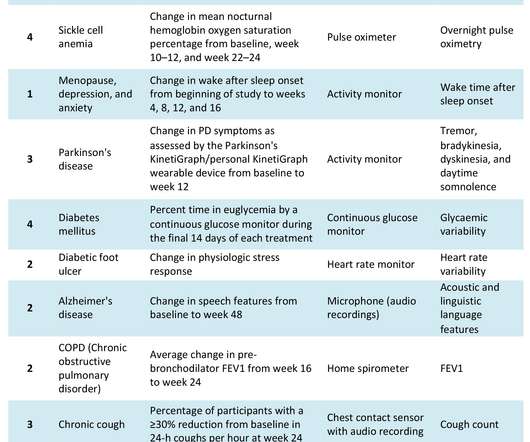
The COVID-19 pandemic has catalysed significant changes in the way pharma develops drugs, particularly in the clinical trial space. Hybrid or decentralised clinical trials (DCTs) have gained traction as technology, infrastructure and knowledge have evolved to support their use. Source: Izmailova et al, 2017.

pharmaphorum
FEBRUARY 7, 2022
The last three years in pharma have seen a growing awareness of and dissatisfaction with a trend that has plagued the industry for its whole life: Nondiverse and unrepresentative clinical trials, which lead to incomplete data and compounded health disparity for minority populations.

World of DTC Marketing
JANUARY 11, 2022
Further, 62% of HCPs said that the most significant area where pharma representatives can add value is, by understanding the needs of HCPs and sharing only relevant content with them to make the interactions more insightful. At the same time, marketing emails are among the top 5 channels used by pharma companies to engage HCPs.

pharmaphorum
FEBRUARY 1, 2022
Across the industry, pharma companies are turning to AI and real-world data to address many of the challenges of running clinical trials. The post Synthetic control arms in clinical trials: Making it happen appeared first on. But what does it take to implement these innovations?
Pharmaceutical Technology
FEBRUARY 2, 2023
Artificial intelligence (AI) continues to be in the news, and as pharma companies invest more to make sure they’re ahead of the game, international agencies have also started to catch up with regulations for AI-led healthcare research. We also explore how AI is being used to design digital twins for clinical trials.

pharmaphorum
FEBRUARY 11, 2021
Pharma companies face many challenges when involving patients in the design of clinical trials – but doing so can have huge benefits further down the line, improving the sustainability and quality of research. Involving patients in discussions.

pharmaphorum
JUNE 14, 2022
Despite more treatment options being a positive, Ben Hargreaves finds that this has raised issues over single-region clinical trials, leading to the FDA rejecting certain treatments and clarifying what is required for approval. Only last month , the US company Merck agreed to pay a potential $1.4 Single-region concerns.

pharmaphorum
MAY 30, 2022
Josh Sackman, president and co-founder of AppliedVR, and Web Sun, president and co-founder of Komodo Health, relay how their newly formed collaboration reshapes the clinical trial process by using data, helping to cut costs and allowing for evaluation of broader patient populations. .
pharmaphorum
NOVEMBER 11, 2022
The lack of diversity in clinical trials has been a topic of debate for decades, but was thrust into the spotlight as the impact of the pandemic on poorer, less educated and ethnically diverse populations became even more apparent. The post IQVIA’s report card for clinical trial diversity: must do better appeared first on.

pharmaphorum
FEBRUARY 9, 2021
Medidata has launched Sensor Cloud, a platform to manage a range of sensor and digital health technology data during clinical trials. In October last year, Medidata bought the digital biomarker firm MC10, adding more clinical analytics and biosensor technology to its cloud offering.

AuroBlog - Aurous Healthcare Clinical Trials blog
APRIL 8, 2024
The Drugs Technical Advisory Board (DTAB) has suggested to the ministry of health and family welfare (MoHFW) to write to the ministry of corporate affairs to mandate the pharma companies to spend almost one per cent of their net profit for providing free medicines in medicine banks as part of the Corporate Social Responsibility (CSR). […]

pharmaphorum
SEPTEMBER 7, 2022
Pharma has a great opportunity ahead of it when it comes to conducting representative clinical trials. The processes used are now seeing huge infrastructure commitments, in order to cement their place in the development of future products and trials. Whilst this is an early area of research, pharma can turn the dial now.

XTalks
DECEMBER 4, 2024
Jupiter Neurosciences, a clinical-stage pharma company specializing in neuroinflammation, made its public market debut with an initial public offering (IPO) on the Nasdaq Capital Market under the symbol “JUNS.” ” The company priced 2,750,000 shares of common stock at $4.00

AuroBlog - Aurous Healthcare Clinical Trials blog
MARCH 17, 2024
The Department of Pharmaceuticals (DoP) has issued a new Uniform Code for Pharmaceutical Marketing Practices (UCPMP) 2024, permitting pharma companies to provide brand reminders such as informational and education items and free samples to medical professionals with restrictions on sample packs and total value.

AuroBlog - Aurous Healthcare Clinical Trials blog
APRIL 11, 2023
The pharma companies’ thrust on R&D to develop better medicines and the demand in hospitals for advanced medical technologies driven by artificial intelligence, remote patient monitoring and wearables are transforming patient care.

AuroBlog - Aurous Healthcare Clinical Trials blog
JANUARY 12, 2023
Indian pharma sees immense value in US FDA’s insistence for aluminum content and labelling recommendations for the Small Volume Parenterals Drug Products and Pharmacy Bulk Packages. Pharma companies in the country have increasingly made a shift to nutritional based prescription and over the counter products.

AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 11, 2023
The Delhi High Court has granted ten days’ time to the Government of India and the nation’s drug regulator to file a counter affidavit on the petitions filed by almost 28 pharma companies against the order prohibiting manufacturing, distribution and sale of 14 FDCs licensed prior to the year 1988, in the beginning of June. […]
XTalks
MAY 6, 2022
In this interview, Xtalks spoke with experts from eClinical Solutions , Katrina Rice, Chief Delivery Officer, Data Services; and Diane Lacroix, Vice President, Clinical Data Management, about clinical data management for modern day digital clinical trials. Data security: The clinical trial data is backed-up and secure.
pharmaphorum
MARCH 28, 2022
Built under a government tender, AION Labs comprises four large international pharma companies – AstraZeneca , Merck, Pfizer , and Teva – and another core partner, the Israel Biotech Fund. Yerushalmi states there are numerous ways AI can support the pharma process – from clinical trials to patient stratification.

World of DTC Marketing
FEBRUARY 7, 2022
At 99% of pharma companies, that's a huge no-no. On top of this, “the company also revealed that the Federal Trade Commission and the Securities and Exchange Commission had opened two new, separate investigations into the troubled biotech. At 99% of pharma companies, that’s a huge no-no.

AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 16, 2023
At least seven more pharma companies and a federation of pharma manufacturers have approached the Delhi High Court against the Central Government’s order in the beginning of June prohibiting manufacturing, distribution and sale of 14 fixed dose combinations (FDCs) licensed prior to the year 1988.

Intouch Solutions
OCTOBER 13, 2023
Pharmaceutical companies can sponsor as well as provide these leaders. Pharmaceutical companies can increase diversity in their clinical trials. Companies can also support pre-existing SDOH research initiatives. Studies should include as detailed SDOH data as possible.

pharmaphorum
MAY 20, 2022
Last year, the company added clinical trials to the list with the launch of CVS Health Clinical Trial Services , an initiative that works with pharma companies on clinical trial recruitment and even hosts trial sites at certain retail locations. Take us back to May 2020.

pharmaphorum
OCTOBER 12, 2022
Walmart is the latest US retail pharmacy giant to announce its intention of disrupting the clinical trials category, with the launch of a new institute that pledges to increase and diversify community access to healthcare research. The post Walmart follows CVS, Walgreens into clinical trial sector appeared first on.

XTalks
MAY 11, 2023
Clinical trials are essential for the development of new treatments for rare diseases, but they can be complex and challenging to execute. Patient input is a crucial component of rare disease clinical trials, as it can provide valuable insights into the patient experience and inform decisions about study design and implementation.

AuroBlog - Aurous Healthcare Clinical Trials blog
FEBRUARY 11, 2025
Yet India needs to develop strategies to mitigate the impact of USAID funding freeze for Indian pharma companies. This is even as a large section of companies exporting to the US noted that even if foreign assistance is […]
The Pharma Data
DECEMBER 20, 2020
UK drug developer Scancell said it has chosen a COVID-19 vaccine candidate, SN14, from more than a dozen potential products to advance to a clinical trial. . SN14 works by targeting the coronavirus’ nucleocapsid and spike proteins to prevent viral replication using the company’s ImmunoBody DNA vaccine technology.

Pharmaceutical Technology
JULY 21, 2022
While large pharma companies have been slow in this area, Merck and GSK are now using 3D printing for clinical trials and manufacturing. Orthopaedic companies have been using 3D printing for their implants for years. 3D bioprinting offers an alternative to animal testing in drug development.

Pharmaceutical Technology
APRIL 13, 2023
With challenges around the R&D, clinical trials, pricing, and market access for these rare disease therapeutics, big pharma often collaborates with specialist biotechnology companies as a method of de-risking the development of gene therapy products.

Pharmaceutical Technology
FEBRUARY 24, 2023
The EU is planning a sweeping revision of its pharma legislation in March, the largest change in 20 years. The overhaul will address drug marketing exclusivity length, pricing, patient access, innovation incentives, antimicrobial resistance, clinical trials, supply chain security and shortages, and environmental impact.

World of DTC Marketing
DECEMBER 1, 2020
Just look at any pharma product website that is nothing but a sales brochure in a majority of cases. ROI drives pharma companies. In the past, pharma product websites had a huge effect on whether a patient asks for an Rx. 5ive: It’s time to update clinical trial information.

Outsourcing Pharma
NOVEMBER 15, 2021
The pharma company has completed enrollment of amyotrophic lateral sclerosis patients in its study investigating drugs like verdiperstat, an oral treatment.

World of DTC Marketing
NOVEMBER 27, 2020
Moderna’s top management has, collectively, sold more than $350 million in stock or investments in the company over the course of this year. Pharma companies have also been capitalizing on the pandemic and positive press releases more broadly.
Pharmaceutical Technology
AUGUST 30, 2022
Only a limited number of international pharma companies, however, operate manufacturing sites in the country. Large Brazilian pharma companies already play a major role in distributing Covid-19 vaccines to all of Latin America. French and Italian manufacturers have two companies in Brazil.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content