The critical role of comprehensive RNA sequencing in liquid biopsy for biomarker discovery and clinical trials
Bio Pharma Dive
JUNE 17, 2024
Elevate precision medicine: dose optimization and immune monitoring through advanced liquid biopsies.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Bio Pharma Dive
JUNE 17, 2024
Elevate precision medicine: dose optimization and immune monitoring through advanced liquid biopsies.

Pharmaceutical Technology
APRIL 2, 2025
The Series B funds will support the launch of a Phase I/II clinical trial for AIR-001 to advance its RNA editing pipeline.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
JULY 29, 2022
Last week, CAMP4 Therapeutics announced the close of a $100 million Series B round , which will be used to advance their regulatory RNA (regRNA)-focused programs. CAMP4’s CSO David Bumcrot PhD tells Pharmaceutical Technology that the company plans to see clinical trials go forward for their urea cycle disorder programs late next year.

Pharmaceutical Technology
JUNE 16, 2023
from Flanders Innovation & Entrepreneurship (VLAIO) to further advance its oncology portfolio targeting RNA. The grant will help Flamingo to support its translational research in a Phase II study of its lead clinical programme, danvatirsen, to treat head and neck squamous cell carcinoma.

Pharmaceutical Technology
MARCH 28, 2023
There is much interest in the industry around RNA-based therapeutics as their utilisation in indications beyond Covid-19 come into focus. In December 2022, BioNTech initiated a Phase I clinical trial of BNT163 – an HSV vaccine candidate. BNT163 is meant to prevent genital lesions caused by HSV-2 and potentially HSV-1.

AuroBlog - Aurous Healthcare Clinical Trials blog
NOVEMBER 1, 2023
The Drug Controller General of India (DCGI) has added in-vitro diagnostic (IVD) medical devices including those for diagnosis of Covid-19, ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) extraction kits, among others into the Class C risk category under the Medical Devices Rules (MDR), 2017.

Pharma Mirror
SEPTEMBER 2, 2021
In-vitro studies conducted at the British virology research laboratory Virology Research Services in London then demonstrated a potent antiviral activity against SARS-CoV-2 and other RNA viruses. The trial was conducted at. The post Codivir Shows Promising Effect Against COVID-19 appeared first on Pharma Mirror Magazine.

XTalks
JANUARY 8, 2024
Whether it’s for a treatment for a chronic ambulatory condition, precision medicine or cell and gene therapy, there is a massive uptick in clinical trial complexity. It’s important to make sure that with this increase in clinical trial complexity, we don’t make our trials overly burdensome to sites or patients,” says Markham.

AuroBlog - Aurous Healthcare Clinical Trials blog
OCTOBER 16, 2022
The husband-and-wife team who co-founded BioNTech, the biotechnology company that partnered with Pfizer to develop an effective messenger-RNA (mRNA) shot against COVID-19, has predicted that a cancer vaccine could be widely available within the next decade.

Pharmaceutical Technology
AUGUST 15, 2024
Clinical trials for the two RNA interference (RNAi) programmes are slated to commence in early 2025.

Pharmaceutical Technology
NOVEMBER 8, 2022
Additionally, Neurophth will oversee the clinical trials and marketing of gene therapy products developed leveraging the new AAV capsids of Cyagen. Under the collaboration, Cyagen is entitled to get research phase and clinical phase milestone payments and sales-based royalty payments totalling over $140m.

AuroBlog - Aurous Healthcare Clinical Trials blog
SEPTEMBER 17, 2024
We are witnessing a revolution in healthcare, driven by advances in genetics, Omics, RNA and CRISPR gene-editing technology, to deliver precision and personalised medicine, said Kiran Mazumdar-Shaw, executive chairperson, Biocon and Biocon Biologics. Biology is opening up new frontiers in medicine.
AuroBlog - Aurous Healthcare Clinical Trials blog
FEBRUARY 11, 2025
Researchers from Arizona State University suggest that stress granules protein and RNA clumps that form around cells in stressful […]

Pharmaceutical Technology
AUGUST 2, 2022
The RNA platform of Samsung Biologics permitted GreenLight to move from mRNA vaccine conceptualisation to the delivery of released clinical trial material in under two years. Following the demonstration at Samsung, the clinical trial of GreenLight’s Covid-19 booster vaccine is anticipated to commence this year.
Pharmaceutical Technology
OCTOBER 14, 2022
In 2016, the companies entered a strategic partnership to develop novel messenger RNA (mRNA) based PCVs. It is currently being assessed in combination with Merck’s anti-PD-1 therapy, Keytruda, as an adjuvant treatment for high-risk melanoma patients in a Phase II clinical trial being conducted by Moderna.

World of DTC Marketing
DECEMBER 29, 2020
Both the Pfizer and Moderna vaccines copied RNA sequence from the virus genome and found a way to manufacture it at scale with high-level processes and quality control. Another consideration is that while in traditional vaccine development, clinical trials are carried out in sequence. Then there are the finances.
STAT News
JANUARY 9, 2023
An experimental RNA treatment reduced liver scarring in half of patients with an inherited disease called alpha-1 antitrypsin deficiency, or AATD, according to results from a mid-stage clinical trial reported Monday by its maker Arrowhead Pharmaceuticals.

XTalks
DECEMBER 4, 2024
Proceeds from the IPO will propel key initiatives, including the Phase II clinical trial of Jotrol in Parkinson’s disease. CAMP4’s RNA-based therapies focus on genetic diseases like urea cycle disorders, while Rapport’s small molecules aim to address epilepsy, pain and bipolar disorder.
Pharmaceutical Technology
JANUARY 19, 2023
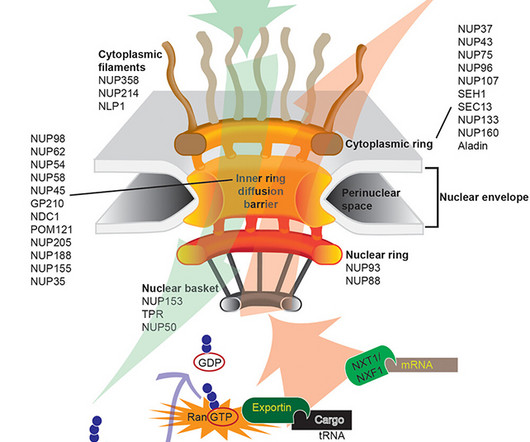
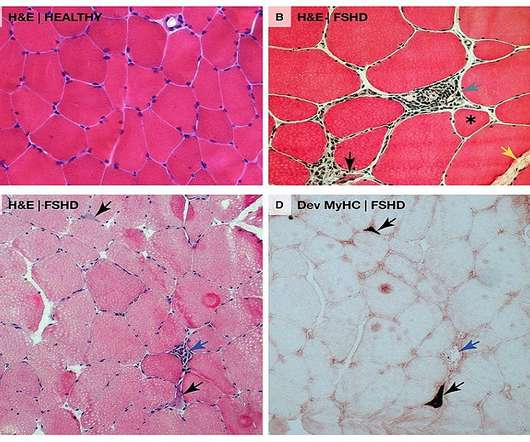
We look forward to working collaboratively with the FDA to bring the first RNA therapy directly targeting DUX4 to patients as quickly as possible.” AOC 1020 is currently being studied in the double-blind, randomised, placebo-controlled Phase I/II FORTITUDE clinical trial in nearly 70 FSHD adult patients.
Pharmaceutical Technology
JUNE 30, 2022
The vaccine leverages self-amplifying RNA (saRNA), which can replicate itself after administration and could be effective at very low doses. At present, the vaccine is being analysed in clinical trials in the US, Brazil and South Korea. HDT Bio CEO Steve Reed said: “Our saRNA vaccine is a game-changer.
Pharmaceutical Technology
JULY 15, 2022
Health Canada has granted approval for the usage of Moderna’s messenger RNA (mRNA) Covid-19 vaccine, Spikevax, in a 25µg two-dose regimen for active immunisation to prevent Covid-19 in children aged six months to five years. The KidCOVE trial was carried out at eight Canadian trial sites involving 414 children aged below five years.

STAT News
JANUARY 30, 2023
It is now possible to treat diseases with gene therapy, antisense oligonucleotides, messenger RNA (mRNA), noncoding RNA (known as small interfering RNA, or siRNA), and other gene-based modalities. New ways of conducting clinical trials have also emerged. The human genome was sequenced in 2003.
Pharmaceutical Technology
MAY 19, 2023
The financing will help to fast-track the development of other in vivo programming candidates into clinical trials. We are pioneering the convergence of immunology and RNA science to build a clinical-stage portfolio of products, starting with the significant unmet needs of cancer patients.

XTalks
APRIL 22, 2022
Flow cytometric receptor occupancy assays are being increasingly used in preclinical and clinical studies. Both the areas of drug development and clinical trials are increasingly using in vitro assays to help determine the efficacy of an investigational therapeutic. What is Flow Cytometry? What is a Receptor Occupancy Assay?

Pharma Mirror
FEBRUARY 2, 2022
Global contract development and manufacturing organisation (CDMO), Recipharm, has today announced the acquisition of GenIbet, a Portuguese CDMO, specialising in the manufacture of biological clinical trial material and novel modalities such as viral vectors, RNA and microbiome.
Pharmaceutical Technology
DECEMBER 11, 2022
In November, the companies announced plans for a Phase I clinical trial of the combined vaccine candidate in healthy adult subjects. The first subject was dosed in the trial, which is designed to evaluate the safety, tolerability and immunogenicity of a nucleoside-modified RNA-based combination vaccine approach.

STAT News
SEPTEMBER 30, 2022
Participants in the study have undergone liver biopsies at baseline and one year to determine if fazirsiran improves disease symptoms, including liver fibrosis.

Pharmaceutical Technology
APRIL 3, 2023
GlobalData, the parent company of Pharmaceutical Technology, outlines a rise in gene therapy trials in 2023, in line with the growing market Fáber describes. According to GlobalData’s Clinical Trials Database, gene therapy trials represent a 0.91% share of all planned trials in 2023, up from just 0.25% in 2014.

Pharmaceutical Technology
NOVEMBER 10, 2022
Olpasiran is entering a Phase III clinical trial to evaluate if the siRNA therapy can reduce cardiovascular event risk in atherosclerotic cardiovascular disease patients with increased Lp(a) levels. . The post Royalty Pharma acquires royalty interest in olpasiran for $250m appeared first on Pharmaceutical Technology.

Pharmaceutical Technology
MAY 15, 2023
The US Food and Drug Administration (FDA) has given orphan drug designation to SiSaf’s siRNA [a double-stranded RNA molecule that is non-coding] therapeutic, SIS-101-ADO, for the treatment of autosomal dominant osteopetrosis type 2 (ADO2), a rare and serious skeletal disorder in children.

pharmaphorum
JULY 20, 2022
Armed with a $100 million second-round financing, CAMP4 Therapeutics is preparing to start the first clinical trial of a drug targeting regulatory RNA (regRNA) molecules that can be used to fine-tune the expression of genes. ” The post CAMP4 raises $100m to take lead RNA drugs into clinic appeared first on.

Worldwide Clinical Trials
JUNE 15, 2022
Further, some patients are still hesitant to go into the clinic, and the prevalence of misinformation from the pandemic has led to greater concerns about therapies like gene therapies and the impact they fear it will have on their RNA/DNA. Working with a centralized IBC/IRB provider can speed up your process. .

Roots Analysis
NOVEMBER 6, 2023
RNA-based therapeutics have completely revolutionized the healthcare segment, greatly influencing the study and treatment of human diseases by conferring precise targeting ability to therapeutic modalities. The below figure presents the distribution of next generation RNA-based therapeutics and RNA-based vaccines, based on type of molecule.

XTalks
FEBRUARY 12, 2025
Current PCSK9 inhibitors on the market include Regenerons monoclonal antibody Praluent (alirocumab), Amgens Repatha (evolocumab) and Novartiss Leqvio (inclisiran), which is a small RNA interference (siRNA) therapy designed to lower LDL-C. We look forward to working with regulators to make lerodalcibep available to patients around the world.
XTalks
OCTOBER 26, 2021
Givlaari reduces ALAS1 levels through RNA interference-mediated gene silencing, which targets ALAS1 mRNA. After participating in a clinical trial evaluating the gene silencing treatment, the sisters told BBC that, “the difference is astronomical, we’re not in pain anymore,” and that they don’t have to rely on opioids for pain relief.
The Pharma Data
JUNE 26, 2021
Sanofi and Translate Bio initiate Phase 1 clinical trial of mRNA influenza vaccine. Th e first clinical trial of a seasonal mRNA flu vaccine candidate is an exciting milestone in our quest for the next generation of inf lu enza vaccines. JUNE 22 , 2021.

Delveinsight
AUGUST 26, 2021
Shape’s RNA editing technologies can modify the RNA sequence, which makes the body’s protein building blocks. This system is designed to deliver RNA editing technology or other payloads directly to particular body areas, such as the nervous system or muscle. and Leila Zegna, director of the Kabuki Syndrome Foundation.

Pharmaceutical Technology
MAY 15, 2023
Rona Therapeutics has entered a partnership with Keymed Biosciences to discover and develop first-in-class siRNA [a double-stranded RNA molecule that is non-coding] therapeutics for glomerulonephritis, also known as severe kidney disease. The programme is expected to proceed towards clinical trials in the first half of 2024.

Worldwide Clinical Trials
AUGUST 14, 2024
Molecular Biomarkers : Encompass various molecules, such as RNA and metabolites, to reflect the physiological state of the cells and their disease pathways. The post The Role of Oncology Biomarkers in Personalizing Hematology Treatment Plans appeared first on Worldwide Clinical Trials.
The Pharma Data
MARCH 11, 2021
Clinical trial to assess safety, immune response and reactogenicity, after preclinical data showed high neutralizing antibody levels. The Companies expect interim results from this trial in the third quarter of 2021. About the Phase 1/2 clinical trial. About previously-published preclinical results.
XTalks
JANUARY 21, 2025
In a clinical trial, participants demonstrated a 98% success rate after six months post implantation, with arteries widened successfully and no stent fractures observed. According to Geneoscopy , ColoSense is the first non-invasive colorectal cancer screening test to provide a dynamic view of disease activity by using RNA biomarkers.
Pharmaceutical Technology
AUGUST 24, 2022
Mode rna has submitted an application to the US Food and Drug Administration (FDA) to obtain emergency use authorization (EUA) for mRNA-1273.222, its BA.4/BA.5 According to the findings, the trial of mRNA-1273.214 met all primary endpoints. 5 Omicron-targeting bivalent booster vaccine for Covid-19. 1 subvariant.
Pharmaceutical Technology
JULY 5, 2022
It was also found in lab experiments that ZCCHC14, a protein that interacts with zinc, attaches to certain part of HAV’s ribonucleic acid (RNA), enabling the virus to recruit TENT4 from the human cell. In addition, the scientists later found that the HAV needs TENT4A/B for its replication.

Pharmaceutical Commerce
FEBRUARY 12, 2024
In clinical trials, bepirovirsen was found to recognize the RNA used by hepatitis B virus to replicate in infected liver cells.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content