The critical role of comprehensive RNA sequencing in liquid biopsy for biomarker discovery and clinical trials
Bio Pharma Dive
JUNE 17, 2024
Elevate precision medicine: dose optimization and immune monitoring through advanced liquid biopsies.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Bio Pharma Dive
JUNE 17, 2024
Elevate precision medicine: dose optimization and immune monitoring through advanced liquid biopsies.

Pharmaceutical Technology
APRIL 2, 2025
The Series B funds will support the launch of a Phase I/II clinical trial for AIR-001 to advance its RNA editing pipeline.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
JULY 29, 2022
Last week, CAMP4 Therapeutics announced the close of a $100 million Series B round , which will be used to advance their regulatory RNA (regRNA)-focused programs. CAMP4’s CSO David Bumcrot PhD tells Pharmaceutical Technology that the company plans to see clinical trials go forward for their urea cycle disorder programs late next year.

Pharmaceutical Technology
MARCH 28, 2023
There is much interest in the industry around RNA-based therapeutics as their utilisation in indications beyond Covid-19 come into focus. In December 2022, BioNTech initiated a Phase I clinical trial of BNT163 – an HSV vaccine candidate. BNT163 is meant to prevent genital lesions caused by HSV-2 and potentially HSV-1.

Pharmaceutical Technology
JUNE 16, 2023
from Flanders Innovation & Entrepreneurship (VLAIO) to further advance its oncology portfolio targeting RNA. The grant will help Flamingo to support its translational research in a Phase II study of its lead clinical programme, danvatirsen, to treat head and neck squamous cell carcinoma.

XTalks
JANUARY 8, 2024
Whether it’s for a treatment for a chronic ambulatory condition, precision medicine or cell and gene therapy, there is a massive uptick in clinical trial complexity. It’s important to make sure that with this increase in clinical trial complexity, we don’t make our trials overly burdensome to sites or patients,” says Markham.

AuroBlog - Aurous Healthcare Clinical Trials blog
NOVEMBER 1, 2023
The Drug Controller General of India (DCGI) has added in-vitro diagnostic (IVD) medical devices including those for diagnosis of Covid-19, ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) extraction kits, among others into the Class C risk category under the Medical Devices Rules (MDR), 2017.

Pharma Mirror
SEPTEMBER 2, 2021
In-vitro studies conducted at the British virology research laboratory Virology Research Services in London then demonstrated a potent antiviral activity against SARS-CoV-2 and other RNA viruses. The trial was conducted at. The post Codivir Shows Promising Effect Against COVID-19 appeared first on Pharma Mirror Magazine.

World of DTC Marketing
DECEMBER 29, 2020
Both the Pfizer and Moderna vaccines copied RNA sequence from the virus genome and found a way to manufacture it at scale with high-level processes and quality control. Another consideration is that while in traditional vaccine development, clinical trials are carried out in sequence. Then there are the finances.

Pharmaceutical Technology
AUGUST 15, 2024
Clinical trials for the two RNA interference (RNAi) programmes are slated to commence in early 2025.

AuroBlog - Aurous Healthcare Clinical Trials blog
OCTOBER 16, 2022
The husband-and-wife team who co-founded BioNTech, the biotechnology company that partnered with Pfizer to develop an effective messenger-RNA (mRNA) shot against COVID-19, has predicted that a cancer vaccine could be widely available within the next decade.
Pharmaceutical Technology
OCTOBER 14, 2022
In 2016, the companies entered a strategic partnership to develop novel messenger RNA (mRNA) based PCVs. It is currently being assessed in combination with Merck’s anti-PD-1 therapy, Keytruda, as an adjuvant treatment for high-risk melanoma patients in a Phase II clinical trial being conducted by Moderna.

Pharmaceutical Technology
AUGUST 2, 2022
The RNA platform of Samsung Biologics permitted GreenLight to move from mRNA vaccine conceptualisation to the delivery of released clinical trial material in under two years. Following the demonstration at Samsung, the clinical trial of GreenLight’s Covid-19 booster vaccine is anticipated to commence this year.

Pharmaceutical Technology
NOVEMBER 8, 2022
Additionally, Neurophth will oversee the clinical trials and marketing of gene therapy products developed leveraging the new AAV capsids of Cyagen. Under the collaboration, Cyagen is entitled to get research phase and clinical phase milestone payments and sales-based royalty payments totalling over $140m.

XTalks
DECEMBER 4, 2024
Proceeds from the IPO will propel key initiatives, including the Phase II clinical trial of Jotrol in Parkinson’s disease. While Jupiter faces financial uncertainties and challenges in scaling operations in competitive markets, it remains committed to refining grant applications for proof-of-concept trials.
Pharmaceutical Technology
JULY 15, 2022
Health Canada has granted approval for the usage of Moderna’s messenger RNA (mRNA) Covid-19 vaccine, Spikevax, in a 25µg two-dose regimen for active immunisation to prevent Covid-19 in children aged six months to five years. The KidCOVE trial was carried out at eight Canadian trial sites involving 414 children aged below five years.
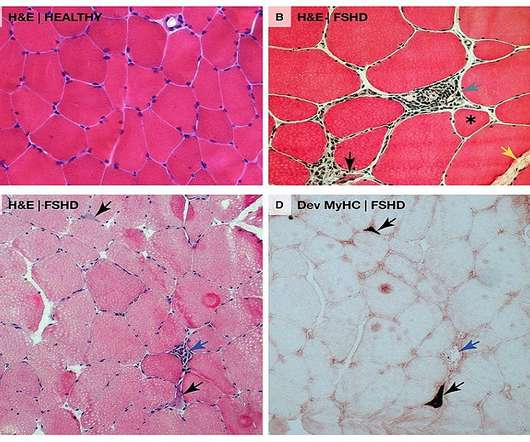
Pharmaceutical Technology
JANUARY 19, 2023
“We look forward to working collaboratively with the FDA to bring the first RNA therapy directly targeting DUX4 to patients as quickly as possible.” AOC 1020 is currently being studied in the double-blind, randomised, placebo-controlled Phase I/II FORTITUDE clinical trial in nearly 70 FSHD adult patients.

AuroBlog - Aurous Healthcare Clinical Trials blog
SEPTEMBER 17, 2024
We are witnessing a revolution in healthcare, driven by advances in genetics, Omics, RNA and CRISPR gene-editing technology, to deliver precision and personalised medicine, said Kiran Mazumdar-Shaw, executive chairperson, Biocon and Biocon Biologics. Biology is opening up new frontiers in medicine.
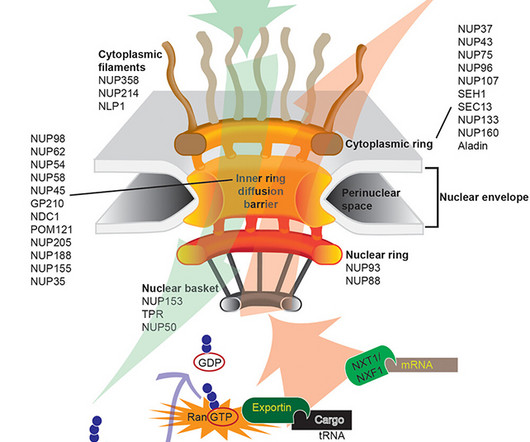
AuroBlog - Aurous Healthcare Clinical Trials blog
FEBRUARY 11, 2025
Researchers from Arizona State University suggest that stress granules protein and RNA clumps that form around cells in stressful […]
Pharmaceutical Technology
JUNE 30, 2022
The vaccine leverages self-amplifying RNA (saRNA), which can replicate itself after administration and could be effective at very low doses. At present, the vaccine is being analysed in clinical trials in the US, Brazil and South Korea. HDT Bio CEO Steve Reed said: “Our saRNA vaccine is a game-changer.
STAT News
JANUARY 9, 2023
An experimental RNA treatment reduced liver scarring in half of patients with an inherited disease called alpha-1 antitrypsin deficiency, or AATD, according to results from a mid-stage clinical trial reported Monday by its maker Arrowhead Pharmaceuticals.
Pharmaceutical Technology
MAY 19, 2023
Myeloid Therapeutics has raised $73m to support the continued clinical development of its lead cell therapy programme, MT-101, in Phase I/II trials for T cell lymphoma. The financing will help to fast-track the development of other in vivo programming candidates into clinical trials.

XTalks
APRIL 22, 2022
Flow cytometric receptor occupancy assays are being increasingly used in preclinical and clinical studies. Both the areas of drug development and clinical trials are increasingly using in vitro assays to help determine the efficacy of an investigational therapeutic. What is Flow Cytometry? What is a Receptor Occupancy Assay?

Pharmaceutical Technology
APRIL 3, 2023
GlobalData, the parent company of Pharmaceutical Technology, outlines a rise in gene therapy trials in 2023, in line with the growing market Fáber describes. According to GlobalData’s Clinical Trials Database, gene therapy trials represent a 0.91% share of all planned trials in 2023, up from just 0.25% in 2014.
Pharmaceutical Technology
DECEMBER 11, 2022
In November, the companies announced plans for a Phase I clinical trial of the combined vaccine candidate in healthy adult subjects. The first subject was dosed in the trial, which is designed to evaluate the safety, tolerability and immunogenicity of a nucleoside-modified RNA-based combination vaccine approach.

STAT News
JANUARY 30, 2023
It is now possible to treat diseases with gene therapy, antisense oligonucleotides, messenger RNA (mRNA), noncoding RNA (known as small interfering RNA, or siRNA), and other gene-based modalities. New ways of conducting clinical trials have also emerged. The human genome was sequenced in 2003.

Pharma Mirror
FEBRUARY 2, 2022
Global contract development and manufacturing organisation (CDMO), Recipharm, has today announced the acquisition of GenIbet, a Portuguese CDMO, specialising in the manufacture of biological clinical trial material and novel modalities such as viral vectors, RNA and microbiome.

pharmaphorum
SEPTEMBER 2, 2020
Spain’s Highlight Therapeutics is attempting to get patients with melanoma to respond to Keytruda by combining with its RNA-based cancer therapy in patients with melanoma who have progressed despite treatment with PD-1 therapies such as Keytruda or BMS’ rival Opdivo (nivolumab). A total of 40 patients are planned to be enrolled.

STAT News
SEPTEMBER 30, 2022
Participants in the study have undergone liver biopsies at baseline and one year to determine if fazirsiran improves disease symptoms, including liver fibrosis.

Worldwide Clinical Trials
JUNE 15, 2022
To discuss your study and how to reduce the chances of receiving a clinical hold, talk with us. . The pandemic has had a lasting impact on the ability of sites to participate in trials, particularly in the US. We need to consider how to use diverse populations in gene therapy trials.

XTalks
FEBRUARY 12, 2025
Current PCSK9 inhibitors on the market include Regenerons monoclonal antibody Praluent (alirocumab), Amgens Repatha (evolocumab) and Novartiss Leqvio (inclisiran), which is a small RNA interference (siRNA) therapy designed to lower LDL-C. Results from the trials demonstrated that lerodalcibep achieved significant reductions in LDL-C levels.

Pharmaceutical Technology
NOVEMBER 10, 2022
Olpasiran is entering a Phase III clinical trial to evaluate if the siRNA therapy can reduce cardiovascular event risk in atherosclerotic cardiovascular disease patients with increased Lp(a) levels. . The post Royalty Pharma acquires royalty interest in olpasiran for $250m appeared first on Pharmaceutical Technology.

Pharmaceutical Technology
MAY 15, 2023
The US Food and Drug Administration (FDA) has given orphan drug designation to SiSaf’s siRNA [a double-stranded RNA molecule that is non-coding] therapeutic, SIS-101-ADO, for the treatment of autosomal dominant osteopetrosis type 2 (ADO2), a rare and serious skeletal disorder in children.
The Pharma Data
JUNE 26, 2021
Sanofi and Translate Bio initiate Phase 1 clinical trial of mRNA influenza vaccine. The trial will evaluate the safety and immunogenicity of a monovalent flu vaccine candidate coding for the hemagglutinin protein of the A/H3N2 strain of the influenza virus. JUNE 22 , 2021.
XTalks
OCTOBER 26, 2021
The Phase III trial results show that Givlaari offers sustainable benefit to AIP patients and confirm its favorable safety profile. Givlaari reduces ALAS1 levels through RNA interference-mediated gene silencing, which targets ALAS1 mRNA. Clinical trial results have shown that Givlaari reduces severe AIP symptoms by 74 percent.
The Pharma Data
MARCH 11, 2021
Clinical trial to assess safety, immune response and reactogenicity, after preclinical data showed high neutralizing antibody levels. The Companies expect interim results from this trial in the third quarter of 2021. About the Phase 1/2 clinical trial.

XTalks
JUNE 4, 2024
In the ever-evolving landscape of pharmaceutical development, the complexity of early phase clinical trials is increasing. The company partners with small and large biopharma and medical device and diagnostic companies to optimize early clinical development through excellence in study design and execution.

Delveinsight
AUGUST 26, 2021
Shape’s RNA editing technologies can modify the RNA sequence, which makes the body’s protein building blocks. This system is designed to deliver RNA editing technology or other payloads directly to particular body areas, such as the nervous system or muscle. and Leila Zegna, director of the Kabuki Syndrome Foundation.

pharmaphorum
JULY 20, 2022
Armed with a $100 million second-round financing, CAMP4 Therapeutics is preparing to start the first clinical trial of a drug targeting regulatory RNA (regRNA) molecules that can be used to fine-tune the expression of genes. ” The post CAMP4 raises $100m to take lead RNA drugs into clinic appeared first on.

The Pharma Data
NOVEMBER 16, 2020
November 16, 2020 — An independent data and safety monitoring board (DSMB) overseeing the Phase 3 trial of the investigational COVID-19 vaccine known as mRNA-1273 reviewed trial data and shared its interim analysis with the trial oversight group on Nov. 37% of trial volunteers are from racial and ethnic minorities.
Pharmaceutical Technology
AUGUST 24, 2022
Mode rna has submitted an application to the US Food and Drug Administration (FDA) to obtain emergency use authorization (EUA) for mRNA-1273.222, its BA.4/BA.5 According to the findings, the trial of mRNA-1273.214 met all primary endpoints. At present, a Phase II/III trial of mRNA-1273.222 is progressing. 1 subvariant.

Worldwide Clinical Trials
AUGUST 14, 2024
Molecular Biomarkers : Encompass various molecules, such as RNA and metabolites, to reflect the physiological state of the cells and their disease pathways. The post The Role of Oncology Biomarkers in Personalizing Hematology Treatment Plans appeared first on Worldwide Clinical Trials.

Roots Analysis
NOVEMBER 6, 2023
RNA-based therapeutics have completely revolutionized the healthcare segment, greatly influencing the study and treatment of human diseases by conferring precise targeting ability to therapeutic modalities. The below figure presents the distribution of next generation RNA-based therapeutics and RNA-based vaccines, based on type of molecule.

The Pharma Data
JANUARY 31, 2021
Adjuvanted S-Trimer COVID-19 vaccine candidates demonstrated favorable safety and tolerability profiles and strong neutralizing immune responses in a phase 1 trial. Clover plans to initiate a global phase 2/3 trial in the first half of 2021 with an interim analysis for vaccine efficacy potentially in the middle of 2021.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content